Highlights
|
Summary
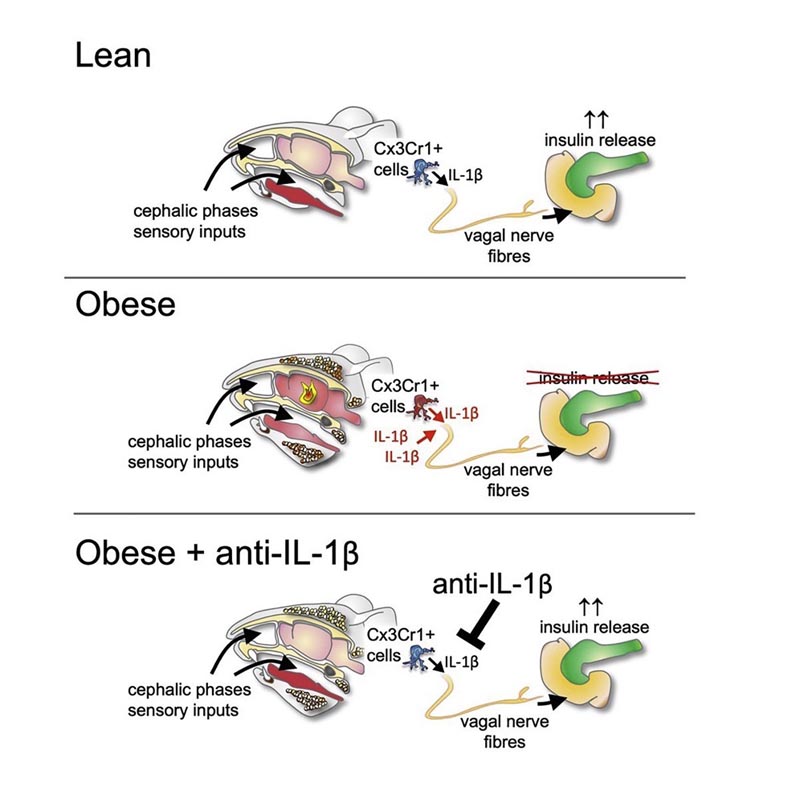
The initial cephalic phase of insulin secretion is mediated by the vagus nerve and is not due to glycemic stimulation of pancreatic β cells. Recently, IL-1β was shown to stimulate postprandial insulin secretion. Here, we describe that this incretin-like effect of IL-1β involves neuronal transmission . Furthermore, we found that insulin release in the cephalic phase was mediated by IL-1β from microglia. Furthermore, IL-1β activated the vagus nerve to induce insulin secretion and regulated the activity of the hypothalamus in response to head stimulation. Notably, insulin release in the cephalic phase was impaired in obesity , in both mice and humans, and in mice this was due to dysregulated IL-1β signaling. Our findings attribute a regulatory role to IL-1β in the integration of nutrient-derived sensory information, subsequent neuron-mediated insulin secretion, and dysregulation of autonomic cephalic phase responses in obesity.
Insulin secretion is regulated by multiple factors. Although the direct effects of glucose action on pancreatic β cells constitute the most prominent stimulus of insulin secretion, other mechanisms, such as neuronal stimulation and incretin hormones , can explain up to 70%–80% of the total response. of insulin to a meal.
Insulin release in the cephalic phase is the consequence of sensory stimuli from food (sight, smell, taste, proprioceptive and tactile stimulation) acting on receptors in the head and oropharynx before the direct stimulant effects of glucose. in β cells.
These stimuli then involve activation of the parasympathetic nervous system and downstream insulin secretion. Cephalic phase responses are essential in the postprandial regulation of glucose homeostasis because a meal that bypasses the oral cavity and therefore cephalic phase responses causes glucose intolerance . Apart from muscarinic receptor signaling in β cells and hypothalamic monoamines, no molecular mediator of insulin release in the cephalic phase has been described to date.
Interleukin 1 beta (IL-1β) regulates the body’s response to tissue damage and pathogens. More recently, IL-1β was shown to be an essential player in both the physiology and pathology of glucose metabolism. Indeed, chronic activation of IL-1β in patients with metabolic syndrome promotes β-cell toxicity and ultimately failure, hallmarks of type 2 diabetes . In contrast to these long-term deleterious effects, acute food intake primes myeloid cells to produce IL-1β, which then contributes to the physiological postprandial stimulation of insulin secretion. However, the mechanisms underlying this incretin-like effect of IL-1β on insulin release remain unknown.
We hypothesize that IL-1β exerts its secretagogue effect on insulin secretion through stimulation of the parasympathetic nervous system . Using genetic and pharmacological models, we found that IL-1β mediates its stimulatory effect on insulin secretion through central muscarinic signaling. To corroborate the neuronal involvement of IL-1β-mediated insulin release, we studied the cephalic phase insulin response as an excellent example of centrally mediated insulin secretion. We identified IL1β as a crucial mediator of this cephalic phase reflex. Originating from long-lived CX3CR1+ myeloid cells, such as microglia, it regulated neuronal activity in the hypothalamus in response to head stimulation. In particular, we found that cephalic phase insulin release is impaired in obesity , both in mice and humans, in an IL-1β-dependent manner in mice. Therefore, IL-1β has a regulatory role in the neuronal stimulation of insulin secretion and in the deregulation of autonomic cephalic phase responses in obesity.
Comments
Even before carbohydrates reach the bloodstream, the mere sight and smell of a meal triggers the release of insulin . For the first time, researchers from the University of Basel and the University Hospital Basel have shown that this insulin release depends on a short-term inflammatory response that occurs under these circumstances. However, in overweight individuals , this inflammatory response is so excessive that it can affect insulin secretion.
Even the anticipation of an upcoming meal triggers a series of responses in the body, perhaps the most familiar of which is saliva in the mouth. But the hormone insulin , which regulates blood sugar, also enters the picture before we even take that first bite. Experts refer to this as the neurally mediated (or cephalic) phase of insulin secretion.
Food stimulates immune defenses
In the past, however, it was unclear how the sensory perception of a meal generated a signal to the pancreas to increase insulin production. Now, researchers from the University of Basel and University Hospital Basel have identified an important piece of the puzzle: an inflammatory factor known as interleukin 1 beta (IL1B), which is also involved in the immune response to pathogens or tissue damage. . The team has reported their findings in the journal Cell Metabolism .
"The fact that this inflammatory factor is responsible for a considerable proportion of normal insulin secretion in healthy individuals is surprising, because it is also involved in the development of type 2 diabetes," explains study leader Professor Marc Donath. , from the Department of Biomedicine and Endocrinology Clinic.
Also known as “adult-onset diabetes ,” this form of diabetes is caused by chronic inflammation that damages the insulin-producing cells of the pancreas, among other things. This is another situation where IL1B plays a key role; in this case, it is produced and secreted in excessively large quantities. With this in mind, clinical studies are now examining whether inhibitors against this inflammatory factor are suitable for use as therapeutic agents for diabetes.
Short-lived inflammatory response
The circumstances are different when it comes to neurally mediated insulin secretion: "The smell and sight of a meal stimulate specific immune cells in the brain known as microglia ," says study author Dr. Sophia Wiedemann, a resident physician. of internal medicine. “These cells briefly secrete IL1B, which in turn affects the autonomic nervous system through the vagus nerve .” This system then transmits the signal to the site of insulin secretion, i.e. the pancreas.
However, in the case of morbid obesity , this phase of neurally mediated insulin secretion is disrupted . Specifically, due to the excessive initial inflammatory response, as explained by doctoral student Kelly Trimigliozzi, who carried out most of the study in collaboration with Wiedemann.
“Our results indicate that IL1B plays an important role in linking sensory information, such as the sight and smell of a meal, to subsequent neural-mediated insulin secretion, and in regulating this connection,” summarizes Marc. Donath.
Conclusions In summary, our findings attribute a regulatory role to IL-1β in the integration of nutrient-derived sensory information, subsequent neuron-mediated insulin secretion, and dysregulation of autonomic cephalic phase responses in obesity. This identifies a neuroimmunological endocrine circuit in the regulation of insulin secretion. |
















