Summary Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a now well-documented disease that commonly arises from a viral infection, but also from other external stressors, such as exposure to agricultural chemicals, other types of infection, surgery or other serious illnesses or stress events. Research has shown that these events produce a systemic molecular inflammatory response and chronic immune activation and dysregulation . What has been more difficult to establish is the hierarchy of physiological responses that give rise to the large number of symptoms that ME/CFS patients experience, and why they do not resolve and typically last a lifetime. Symptom severity frequently fluctuates throughout relapse recovery periods, with symptoms of neuroinflammation centered in the brain, loss of homeostatic control, "brain fog" affecting cognitive ability, lack of restful sleep, and lack of responsiveness. deficient even at minor stresses. How these ME/CFS brain effects develop from the initiating external effector, whether a virus or another cause, is not well understood, and that is what our article aims to address. We hypothesize that after the initial stressful event, subsequent systemic pathology translocates to the brain via neurovascular pathways or through a dysfunctional blood-brain barrier (BBB), resulting in chronic neuroinflammation and leading to sustained disease with recovery cycles from chronic relapses. Signaling through recognized pathways from the brain to the body’s physiology is probably part of the process by which the disease cycle is maintained in the peripheral system and why healing does not occur. In contrast, long COVID (post-COVID-19 condition) is a very recent ME/CFS-like illness arising from the single pandemic virus, SARS-CoV-2. We believe that the ongoing ME/CFS-like effects of Long COVID are arising by very similar mechanisms involving neuroinflammation, but likely with some unique signaling, as a result of the pathology of the initial SARS-CoV-2 infection. The fact that there are very similar symptoms in both ongoing diseases, despite the diversity in the nature of the initial stressors, supports the concept of a similar dysfunctional CNS. |
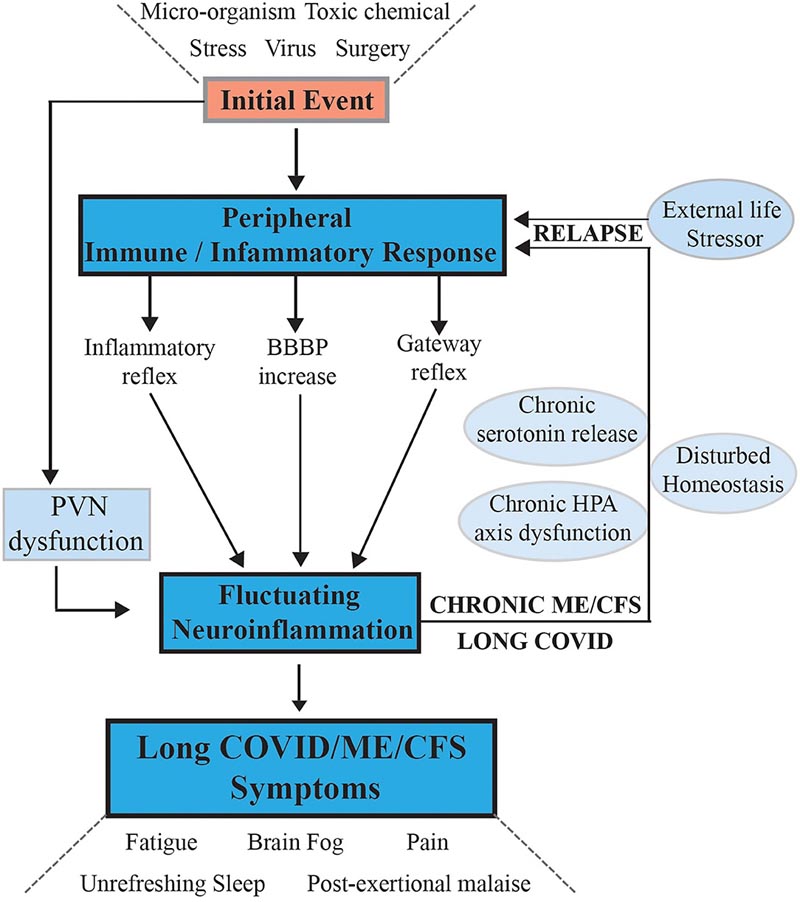
Figure 1. Hypothesis and model for the onset of ME/CFS and its progression to a sustained chronic disease with phases of relapse/partial recovery: signaling pathways between the CNS and the periphery that maintain the disease. After an initial external stressful event, systemic immune/inflammatory responses are activated, these are communicated to the CNS through inflammatory and gateway reflexes and possibly an increase in BBB permeability. Neuroinflammation is activated by affecting the stress center within the PVN of the hypothalamus and leads to a wide range of neurological symptoms that feed back to the periphery through disruption of homeostasis and the stress-activated HPA axis that becomes dysfunctional with chronic activation. Systemic physiology and molecular homeostasis are then chronically affected through important cellular functions such as mitochondrial energy production, metabolic activity and the continuation of immune/inflammatory reactions. External life stressors that fuel a disturbed PVN not only maintain ME/CFS but also act to precipitate relapses.
Comments
Researchers have discovered how post-viral fatigue syndromes , including long COVID, become life-changing illnesses and why patients suffer frequent relapses.
As a common result of a viral infection, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is known to cause brain-focused symptoms of neuroinflammation, loss of homeostasis, mental confusion, lack of restful sleep and poor response to even mild stress situations.
Long-COVID has similar effects on people and is also thought to be caused by neuroinflammation.
Lead author Emeritus Professor Warren Tate, from the Department of Biochemistry at the University of Otago, says how these debilitating brain effects develop is not well understood.
In a study published in Frontiers in Neurology , he and colleagues at Otago, Victoria University of Wellington and the University of Technology Sydney developed a unifying model to explain how the brain-focused symptoms of these diseases are maintained across a long period of time. brain-body connection .
They propose that, after an initial viral infection or stressful event, subsequent systemic pathology is translocated to the brain via neurovascular pathways or through a dysfunctional blood-brain barrier. This results in chronic neuroinflammation , leading to sustained disease with recovery cycles from chronic relapses.
The model proposes that healing does not occur because a signal continually circulates from the brain to the body, causing the patient to relapse.
Creating this model is not only important for the “huge research effort ahead,” but also to provide recognition to ME/CFS and long COVID patients.
“These diseases are very closely related, and it is clear that the biological basis of Long COVID is unequivocally connected to the original COVID infection, so there should no longer be any debate or doubt about the fact that post-viral fatigue syndromes such as ME/CFS are biologically based and involve highly altered physiology,” says Professor Emeritus Tate. This work will allow medical professionals to develop the best evidence-based knowledge of these diseases and best management practices.
“Patients need appropriate affirmation of their biologically based illness and help to mitigate the distressing symptoms of these life-changing syndromes that are difficult for patients to manage on their own.
“This work highlighted that there is a susceptible subset of people who develop such syndromes when exposed to severe stress, such as COVID-19 infection, or Epstein Barr glandular fever virus, or in some people with vaccines that are interpreted like severe stress.
“What should be a transient inflammatory/immune response in the body to clear infection, develop immunity, and control physiological stress, becomes chronic and therefore the disease persists.”