Summary Some people can endure persistent and debilitating symptoms for many months after an initial severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, the factors underlying these health problems, called long Covid, are not well understood. By comparing the blood of patients with confirmed SARS-CoV-2 infection with that of uninfected controls, Cervia-Hasler et al. found that patients experiencing long COVID had changes in blood serum proteins indicating activation of the immune system’s complement cascade, impaired coagulation, and tissue injury . At the cellular level, it was linked to aggregates comprising monocytes and platelets . These findings provide a resource of potential biomarkers for diagnosis and may inform treatment directions. |
Acute severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causes diverse clinical phenotypes, ranging from asymptomatic to life-threatening COVID-19. About 5% of all infected people do not recover from the acute illness but develop long-term complications, called long Covid. Current hypotheses about the factors that contribute to long Covid include tissue damage, viral reservoirs, autoimmunity and persistent inflammation. Currently there are no diagnostic tests or therapeutic solutions for affected patients.
Methodology
We followed 39 healthy controls and 113 COVID-19 patients for up to 1 year after initial confirmation of acute SARS-CoV-2 infection to identify biomarkers associated with long Covid. In the 6-month follow-up , 40 patients had symptoms of long Covid.
Repeat clinical assessments were combined with blood draws, resulting in a total of 268 longitudinal blood samples. We measure >6500 proteins in serum using proteomics . The main candidate biomarkers were identified using computational tools and evaluated experimentally.
Results
Long Covid patients showed greater complement activation during acute illness, which also persisted at 6-month follow-up. The complement system is part of the innate immune system and contributes to immunity and homeostasis by attacking pathogens and damaged cells, among other functions. Interestingly, blood complement levels normalized in long Covid patients who recovered before 6 months of follow-up.
The complement system can be activated by various triggers, resulting in the formation of the terminal complement complex (TCC), formed by complement components C5b-9. These complexes can integrate into cell membranes and induce cell activation or lysis. Long Covid patients showed an unbalanced formation of the terminal complement complex (TCC), marked by an increase in soluble C5bC6 complexes and a decrease in the levels of C7-containing TCC formations that can be incorporated into cell membranes. This suggested increased insertion of CBT membranes in long Covid patients, which contributed to tissue damage.
Consequently, long Covid patients showed elevated markers of tissue injury in the blood and a thromboinflammatory signature , characterized by markers of endothelial activation, such as von Willebrand factor (vWF) and red blood cell lysis. Low antithrombin III levels in long Covid patients were accompanied by signs of increased cleavage by thrombin, a driver of terminal complement complex (TCC) formation.
Furthermore, patients with long Covid had markers of platelet activation and elevated monocyte-platelet aggregates at the 6-month follow-up, particularly in cases where long Covid persisted for 12 months or more. These patients also showed signs of antibody-mediated activation of the classical complement pathway, which was associated with increased anti-CMV (cytomegalovirus, also known as human herpesvirus 5) and anti-EBV (herpesvirus 5) immunoglobulin G (IgG) antibodies. Epstein-Barr).
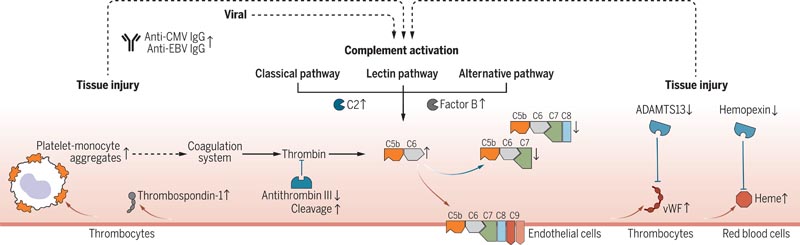
Figure : Pathomechanistic model of long Covid. Complement-mediated thromboinflammation model, showing an increase and decrease in biomarkers (up arrows and down arrows, respectively) measured at 6 months follow-up in patients with persistent long Covid symptoms compared to recovered COVID-19 patients. 19 and healthy controls. Measurements were performed using proteomics, spectral flow cytometry, single-cell transcriptomics, high-throughput antibody measurements, and targeted assays. Red arrows mark activating protein interactions and blue arrows mark inhibitory protein interactions. Dashed arrows connect changes in different biological pathways.
Conclusions Our data suggest that active long Covid is accompanied by a blood protein signature marked by increased complement activation and thromboinflammation, including activated platelets and markers of red blood cell lysis. Tissue injury may also be mediated by complement and, in turn, activate the complement system. Additionally, complement activation can be driven by antigen-antibody complexes, involving autoantibodies and antibodies to herpesviruses, as well as interference with a dysregulated coagulation system. In addition to providing a foundation for new diagnostic solutions, our work supports clinical research into complement modulators for patients suffering from long Covid. |