Some adult patients with immunoglobulin A tissue transglutaminase antibody (IgA-tTG) levels at or above 10 times the upper limit of normal (ULN) and a moderate-to-high pre-test probability of celiac disease could be diagnosed without undergoing invasive endoscopy and duodenal biopsy, according to a new study.
Current international guidelines recommend duodenal biopsies to confirm celiac disease in adult patients. However, growing evidence suggests that invasive procedures may not be necessary, the authors wrote.
“Our study confirms the high accuracy of serology-based diagnosis of celiac disease in selected adult patients,” said Mohamed G. Shiha, MBBCh, MRCP, lead author and clinical researcher in gastroenterology at Sheffield Teaching Hospitals in the UK.
“This non-biopsy approach could lead to a shorter time to diagnosis, increased patient satisfaction, and reduced healthcare costs,” he added.
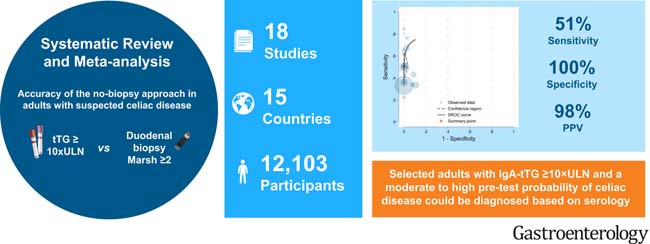
Current international guidelines recommend duodenal biopsies to confirm celiac disease in adult patients. However, increasing evidence suggests that levels of immunoglobulin A tissue transglutaminase antibodies (IgA-tTG) ≥10 times the ULN can accurately predict celiac disease, eliminating the need for biopsy. We conducted a systematic review and meta-analysis to evaluate the accuracy of the non-biopsy approach for confirming celiac disease in adults.
Systematic searches were conducted in MEDLINE, EMBASE, Cochrane Library, and Web of Science from January 1998 to October 2023 for studies reporting the sensitivity and specificity of IgA-tTG ≥10×ULN versus duodenal biopsies (Marsh grade ≥2) in adults suspected of having celiac disease. A bivariate random-effects model was used to calculate pooled estimates of sensitivity, specificity, and positive and negative likelihood ratios. Positive and negative likelihood ratios were used to calculate the positive predictive value of the non-biopsy approach at various pre-test probabilities of celiac disease. The methodological quality of included studies was assessed using the QUADAS-2 tool. This study was registered in PROSPERO, number CRD42023398812.
A total of 18 studies with 12,103 participants from 15 countries were included. The pooled prevalence of biopsy-confirmed celiac disease in the included studies was 62% (95% confidence interval [CI], 40%–83%). The proportion of patients with IgA-tTG ≥10×ULN was 32% (95% CI, 24%–40%).
The pooled sensitivity of IgA-tTG ≥10×ULN was 51% (95% CI, 42%–60%), and the pooled specificity was 100% (95% CI, 98%–100%).
The pooled area under the receiver operating characteristic curve was 0.83 (95% CI, 0.77–0.89).
The positive predictive value of the non-biopsy approach for identifying patients with celiac disease was 65%, 88%, 95%, and 99% if the celiac disease prevalence was 1%, 4%, 10%, and 40%, respectively.
The heterogeneity between studies was moderate (I² = 30.3%), and additional sensitivity analyses did not significantly alter our findings. Only one study had a low risk of bias across all domains.
The results of this meta-analysis suggest that selected adult patients with IgA-tTG ≥10×ULN and a moderate-to-high pre-test probability of celiac disease could be diagnosed without undergoing invasive endoscopy and duodenal biopsy.
Due to the higher accuracy of serological tests, pediatric guidelines have adopted a non-biopsy approach, the authors wrote. Children with IgA-tTG ≥10×ULN and positive endomysial antibodies (EMA) can be diagnosed with celiac disease without the need for a biopsy.
However, the non-biopsy approach remains controversial for diagnosing adult patients and requires further studies, the authors noted. They cited one limitation: all included studies were conducted in secondary and tertiary care settings and excluded patients with known celiac disease or those on a gluten-free diet, so the results may not be generalizable to primary care settings.
Moreover, relying solely on serological tests could lead to potential false positives, unnecessary dietary restrictions, and negative effects on patients’ quality of life, the authors added.
At the same time, duodenal biopsy may not always be accurate due to inadequate sampling, which could result in falsely negative histology. The authors noted that the non-biopsy approach could mitigate this potential risk.
“This study systematically compiles the growing data supporting the accuracy of antibody testing for diagnosing celiac disease,” said Benjamin Lebwohl, MD, AGAF, professor of medicine and epidemiology at Columbia University Medical Center and director of clinical research at the Columbia Celiac Disease Center in New York. Dr. Lebwohl was not involved in this study.
“Historically, we have relied on duodenal biopsy to confirm the diagnosis of celiac disease, and biopsy will continue to play a central role in most cases for the foreseeable future,” he said. “But as we refine our understanding of antibody testing, we may one day accept or even recommend a non-biopsy approach in selected patients.”
















