Key Points from a Review on the Long-Term Safety of Low-Dose Colchicine for Atherosclerosis
FDA Approval: Colchicine 0.5 mg daily is FDA-approved for secondary prevention in patients with coronary artery disease (CAD).
Clinical Trials: Recommendations are based on the LoDoCo2 and COLCOT trials, which showed a 31% reduction in cardiovascular events in patients with clinically stable atherosclerosis and a 23% reduction in patients with recent myocardial infarction.
Doctor´s Experience: Most doctors are familiar with colchicine for treating gout and pericarditis, which involve higher doses often associated with side effects.
Hesitation in Usage: Some doctors hesitate to prescribe the low dose recommended for CAD due to concerns about potential side effects.
Long-Term Analysis: A review of 20 years of continuous colchicine use for various conditions found that a daily dose of 0.5 mg in appropriate patients has side effects similar to a placebo, including no significant issues with myelosuppression, myotoxicity, or cancer.
Infection Risk: There is minimal evidence that colchicine increases the risk of serious or fatal infections.
Common Side Effects: Patients may experience mild diarrhea when starting low-dose colchicine, but it usually resolves quickly.
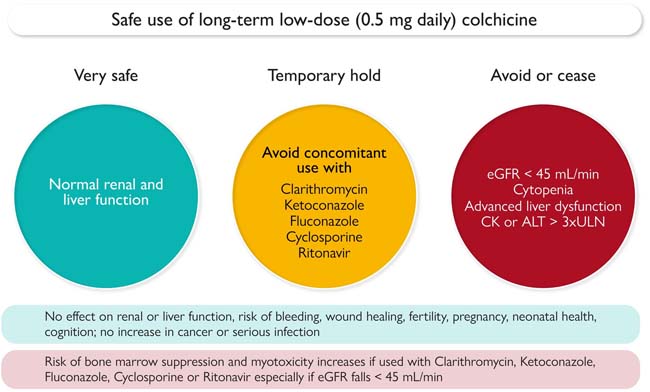
Safety Profile: Low-dose colchicine does not negatively affect bleeding, wound healing, fertility, or pregnancy, even in patients taking statins.
Contraindications: Low-dose colchicine should not be used in patients with an estimated glomerular filtration rate <45 ml/min/1.73 m², cytopenia, advanced liver dysfunction, or elevated creatine kinase or alanine transaminase levels (>3 times the upper limit of normal).
Drug Interactions: Colchicine should be discontinued in patients taking strong CYP3A4 inhibitors or P-glycoprotein inhibitors such as clarithromycin, ketoconazole, fluconazole, cyclosporine, or ritonavir.
General Safety: Long-term use of low-dose colchicine is very safe in patients with normal kidney and liver function.
Broader Use: The clear safety profile of low-dose colchicine in appropriate patients suggests it should be more widely used for secondary prevention of CAD in patients with normal renal and hepatic function.
Source: Low-dose colchicine for atherosclerosis: long-term safety. Stefan Mark Nidorf, Eldad Ben-Chetrit, Paul M Ridker. European Heart Journal, ehae208, https://doi.org/10.1093/eurheartj/ehae208 Published: 10 April 2024
Summary
Low-dose colchicine (0.5 mg daily) is now FDA-approved for secondary prevention in patients with coronary artery disease and is expected to be increasingly prescribed in clinical practice. This state-of-the-art review collected data from contemporary systematic reviews of case reports, drug registries, and placebo-controlled trials to assess specific safety concerns related to the continuous use of colchicine in various clinical settings. This information aims to inform doctors, pharmacists, and patients about the absolute risks of long-term low-dose colchicine use, including among individuals taking statin therapy.
Based on the collective data, the review concludes that apart from mild diarrhea at the start of colchicine, which typically resolves within a week for most patients, long-term low-dose colchicine use is well tolerated and very safe. It does not adversely affect kidney, liver, or cognitive function, nor does it impact bleeding, wound healing, fertility, or pregnancy. Additionally, it does not increase the risks of cancer, serious infections, or cause-specific mortality. When appropriately prescribed to patients without significant renal or hepatic impairment, reports of myelosuppression, myotoxicity, and serious drug interactions are rare and no more frequent than with placebo, even among patients taking statins.
Doctors, pharmacists, and patients can be confident that, in the absence of significant renal or hepatic impairment, continuous low-dose colchicine use is safe for patients with atherosclerosis to reduce cardiovascular risk.
















