Lecanemab in early Alzheimer’s disease
Background
The accumulation of soluble and insoluble aggregated beta-amyloid (Aβ) can initiate or potentiate pathological processes in Alzheimer’s disease. Lecanemab, a humanized IgG1 monoclonal antibody that binds with high affinity to soluble Aβ protofibrils, is being tested in people with early Alzheimer’s disease.
Methods
We conducted an 18-month, multicenter, double-blind, Phase 3 trial involving people aged 50 to 90 years with early Alzheimer’s disease (mild cognitive impairment or mild dementia due to Alzheimer’s disease) with evidence amyloid on positron emission tomography (PET) or cerebrospinal fluid analysis.
Participants were randomly assigned in a 1:1 ratio to receive intravenous lecanemab (10 mg per kilogram of body weight every 2 weeks) or placebo. The primary endpoint was the change from baseline to 18 months in the Clinical Dementia Rating-Sum of Boxes (CDR-SB; range, 0 to 18, with higher scores indicating greater deterioration) score.
Key secondary endpoints were change in amyloid burden on PET, score on the 14-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog14; range, 0 to 90;
Results
A total of 1,795 participants were enrolled , with 898 assigned to receive lecanemab and 897 to receive placebo. The mean CDR-SB score at baseline was approximately 3.2 in both groups.
The adjusted least squares mean change from baseline to 18 months was 1.21 with lecanemab and 1.66 with placebo (difference, −0.45; 95% confidence interval [CI], −0.67 a −0.23; P < 0.001).
In a substudy with 698 participants, there were greater reductions in brain amyloid burden with lecanemab than with placebo (difference, -59.1 centiloids; 95% CI, -62.6 to -55.6).
Other mean differences between the two groups in the change from baseline in favor of lecanemab were as follows: for the ADAS-cog14 score, −1.44 (95% CI, −2.27 to −0.61; P < 0.001); for ADCOMS, −0.050 (95% CI, −0.074 to −0.027; P<0.001); and for the ADCS-MCI-ADL score, 2.0 (95% CI, 1.2 to 2.8; p<0.001).
Lecanemab caused infusion-related reactions in 26.4% of participants and amyloid-related imaging abnormalities with edema or effusion in 12.6%.
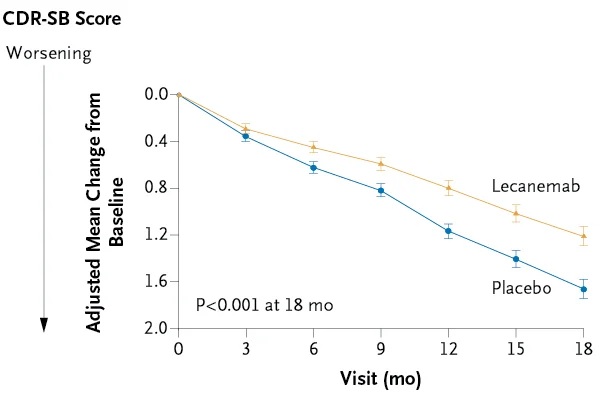
Conclusions Lecanemab reduced amyloid markers in early Alzheimer’s disease and resulted in moderately less decline in measures of cognition and function than placebo at 18 months, but was associated with adverse events . Longer trials are warranted to determine the efficacy and safety of lecanemab in early Alzheimer’s disease. |
(Funded by Eisai and Biogen; Clarity AD ClinicalTrials.gov number, NCT03887455. opens in new tab.)















