A prostate cancer risk tool developed by UK researchers could help better target screening and diagnosis, a study suggests.
Aim
Prostate cancer (PCa) is highly hereditary. There is currently no validated PCa risk model. Therefore, we sought to develop a genetic risk model that can provide personalized predicted PCa risks based on known moderate- to high-risk pathogenic variants, common low-risk genetic variants, and explicit family history of cancer, and to externally validate the model. in an independent prospective cohort.
Materials and methods
We developed a risk model using a relative cohort composed of individuals from 16,633 families with PCa determined in the United Kingdom between 1993 and 2017 from the United Kingdom Prostate Cancer Genetic Study, and a complex segregation analysis adjusted for check. The model was externally validated in 170,850 unaffected men (7624 incident PCa) recruited between 2006 and 2010 for the independent UK Biobank prospective cohort study.
Results
The most parsimonious model included the effects of pathogenic variants in BRCA2 , HOXB13 , and BRCA1 , and a polygenic score based on 268 common low-risk variants.
Residual familial risk was modeled using a hypothesized recessively inherited variant and a polygenic component whose standard deviation decreased linearly with age. The model predicted familial risks that were consistent with those reported in previous observational studies.
In the validation cohort, the model discriminated well between unaffected men and men with incident PCas within 5 years (C-index, 0.790; 95% CI, 0.783 to 0.797) and 10 years (C-index, 0.772; 95% CI, 0.783 to 0.797). 95%, 0.768 to 0.777). The 50% of men with the highest predicted risks captured 86.3% of PCa cases over 10 years.
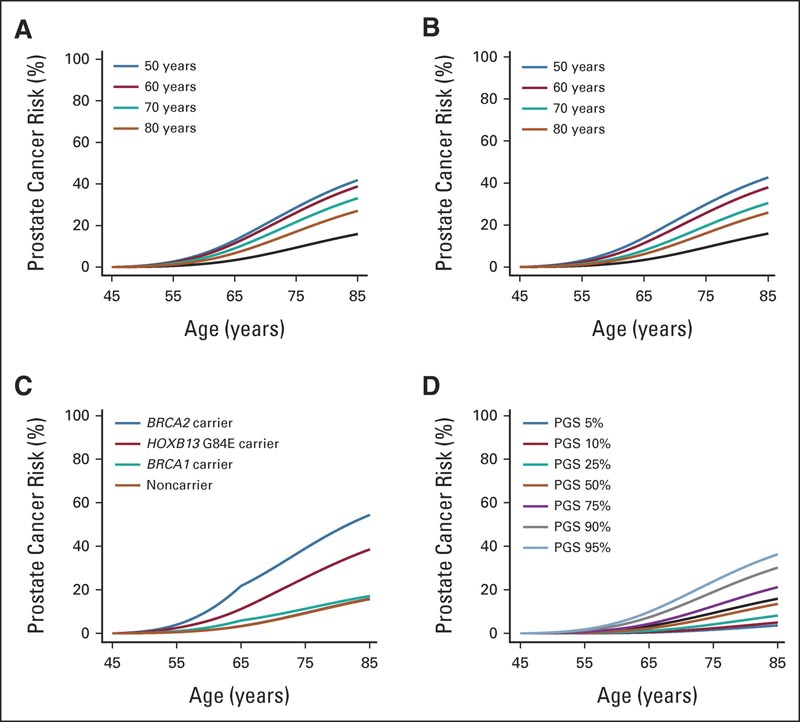
Predicted cumulative prostate cancer risks for a 45-year visit and by (A) father’s age at prostate cancer diagnosis, (B) brother’s age at prostate cancer diagnosis, (C) pathogenic variants or (D) polygenic score percentile. For comparison, all graphs show the average risk of the population (black curve). It was assumed that the consultants and siblings were born after 1960 and that the parents were born in the 1930-1939 birth cohort.
Conclusions
To our knowledge, this is the first validated risk model that offers personalized PCa risks. The model will help counsel men concerned about their risk and may facilitate future risk-stratified population screening approaches.
Comments
Research is now being planned into how the CanRisk-Prostate tool , which combines information about genetic variants and family history of prostate cancer to provide personalized cancer risk, could be used in primary care, researchers at the University of Massachusetts said. Cambridge.
The comprehensive score uses rare but moderate-to-high-risk inherited defective versions of the BRCA2, HOXB13 and possibly BRCA1 genes along with 268 other more common but low-risk variants, along with detailed family cancer history.
The model was built using genetic and family history data from nearly 17,000 families affected by prostate cancer and was validated using data from the UK Biobank study of 170,000 men.
Reporting the results in the Journal of Clinical Oncology , the researchers said the predicted risk was higher for men whose father had been diagnosed with prostate cancer: 27% if the father had been diagnosed at an older age (80 years) but as high as 42% if the father was diagnosed at age 50.
In terms of genetic variation, the researchers found that 54% of men with an alteration in the BRCA2 gene would develop prostate cancer, but among those with faults in the BRCA2 gene, the risks were substantially lower if they also had a small amount of low-risk variants, but much higher if they also had a large number of low-risk variants.
It will be the combination of family history, rare and common genetic variations that will allow for a much more accurate personalized risk score to guide screening and diagnostic decisions, they concluded.
Testing the model on the Biobank population, of which 7,600 developed prostate cancer in the decade after joining the study, the researchers found that 86% of those diagnosed with the condition were in the men’s half with the highest anticipated risks.
Once more studies are done, GPs could use the tool to advise men about their risk, but also for better direct PSA testing or the decision to perform further testing in those with elevated PSA.
Professor Antonis Antoniou, from the Department of Public Health and Primary Care at the University of Cambridge, said: "What we need is a way to identify men who are most at risk, allowing us to target screening and diagnosis where more are needed while reducing harm for those men who are at low risk of contracting the disease.’
The researchers said that over the next year they would incorporate the risk model into the CanRisk tool, already widely used, to aid risk-based clinical management of men seen in family cancer clinics.
And the team will also include more data in the CanRisk-Prostate tool in men of non-European ethnicities to expand its applicability.
Co-author Professor Ros Eeles from the Institute of Cancer Research, London, added: "This is an important step forward as it will allow doctors to talk to men about their individual risk of prostate cancer based on the most accurate computer model." till the date".
Context Key objective Can a genetic risk model that uses information on all known genetic susceptibility variants for high-, moderate-, and low-risk prostate cancer, along with family history (FH) information for residual cancer, accurately predict the risk of men from developing prostate cancer in the future? Generated Knowledge We developed a genetic risk model using data from 16,633 families with prostate cancer. The model uses data on rare pathogenic variants in the moderate-to-high-risk genes BRCA2, HOXB13, and BRCA1, a polygenic score based on 268 common low-risk variants, and detailed cancer FH to predict future risks. The risk model predicted incident prostate cancers in an independent cohort of 170,850 men followed prospectively with high discrimination and good calibration. The majority, 86%, of incident prostate cancers occurred among the half of men with the highest predicted risks. Relevance This multifactorial risk prediction model includes genetic variant data and FH information and will be beneficial for counseling men in family cancer clinics and guide future research evaluating risk-stratified population screening approaches. In conclusion , to our knowledge, this multifactorial risk prediction model is the first to incorporate the effects of currently known moderate- to high-risk and low-risk PCa risk variants along with detailed FH information. The model predicts consistent familial risks and shows good discrimination and calibration in an independent prospective validation cohort. The model will be beneficial for counseling men in family cancer clinics and may form the basis for future research evaluating risk-stratified population screening approaches. |
















