| Introduction |
Long COVID (sometimes called “post-acute COVID-19 sequelae” ) is a multisystem condition comprising often severe symptoms following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
This entity is associated with all ages and severities of the disease in the acute phase, with the highest percentage of diagnoses between 36 and 50 years of age.
Most cases are found in non-hospitalized people with mild acute illness, as this population represents the majority of overall COVID-19 cases.
Hundreds of biomedical findings have been documented with many patients experiencing dozens of symptoms in multiple organ systems ( Fig. 1 ). Long COVID encompasses multiple adverse outcomes, with common new-onset conditions including cardiovascular, thrombotic and cerebrovascular diseases, type 2 diabetes, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), and dysautonomia, especially postural orthostatic tachycardia (STOP) syndrome. . Symptoms can last for years and particularly in cases of new-onset myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia, it is expected to be lifelong.
Several hypotheses have been suggested for its pathogenesis, including persistent reservoirs of SARS-CoV-2 in tissues; immune dysregulation with or without reactivation of underlying pathogens, including herpes viruses such as Epstein-Barr virus (EBV) and human herpes virus 6 (HHV-6); impacts of SARS-CoV-2 on the microbiota, including the virome; autoimmunity and priming of the immune system through molecular mimicry; microvascular blood coagulation with endothelial dysfunction; and dysfunctional signaling in the brainstem and/or vagus nerve ( Fig.2 ).
Risk factors potentially include female sex, type 2 diabetes, EBV reactivation, the presence of specific autoantibodies, connective tissue disorders, attention deficit hyperactivity disorder, chronic urticaria, and allergic rhinitis, although a third of People with long COVID have no identified pre-existing conditions.
This review explores the current knowledge base on long COVID, as well as misconceptions and areas where additional research is needed.
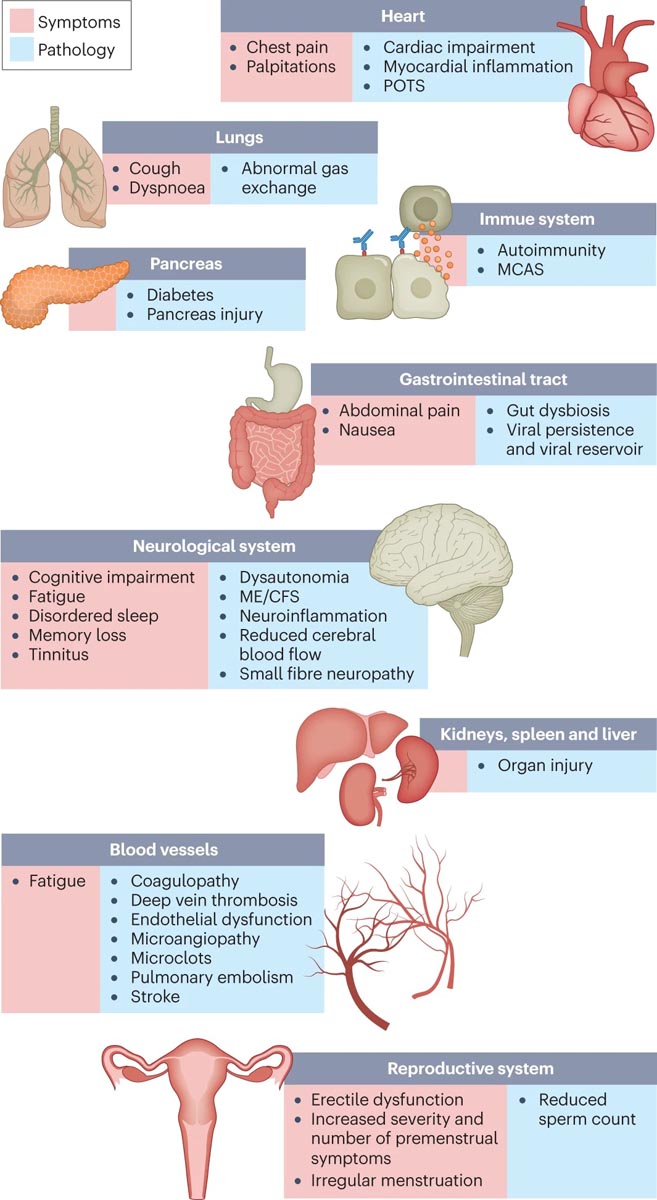
Figure 1. Prolonged COVID symptoms and the impacts on numerous organs with different pathologies. The impacts of long COVID on numerous organs with a wide variety of pathologies are shown. The presentation of pathologies often overlaps, which can exacerbate management challenges. MCAS, mast cell activation syndrome; ME/CFS, myalgic encephalomyelitis/chronic fatigue syndrome; POTS, postural orthostatic tachycardia syndrome.
Relevant discoveries
Immunology and virology
Studies looking at immune dysregulation in people with long COVID who had mild acute COVID-19 have found T cell alterations that persist for at least 13 months. Studies have also reported highly activated innate immune cells, lack of naïve T and B cells, and elevated expression of type I and type III interferons (interferon-β (IFNβ) and IFNλ1), persisting for at least 8 months.
Multiple studies have found elevated levels of autoantibodies in patients with long COVID, including autoantibodies against ACE2 (the receptor for SARS-CoV-2 entry), β2-adrenoceptor, muscarinic M2 receptor, angiotensin II AT1 receptor, and the angiotensin 1 receptor. –7 MORE.
Reactivated viruses, including EBV and HHV-6, have been found in long COVID patients (and have been identified in ME/CFS), which lead to mitochondrial fragmentation and severely affect energy metabolism.
Vascular problems and organ damage
Although COVID-19 was initially recognized as a respiratory disease, SARS-CoV-2 has the ability to damage many organ systems. The damage that has been demonstrated in various tissues has been predominantly attributed to the immune-mediated response and inflammation, rather than direct infection of cells by the virus. Disruption of the circulatory system includes endothelial dysfunction and subsequent downstream effects, and increased risks of deep vein thrombosis, pulmonary embolism, and hemorrhagic events.
Microclots detected in both acute COVID-19 and long COVID contribute to thrombosis and are an attractive diagnostic and therapeutic target. Long-term changes in the size and stiffness of blood cells have also been found in long-COVID cases, with the potential to affect oxygen delivery. A long-lasting reduction in vascular density, specifically affecting small capillaries, was found in long COVID patients compared to controls, 18 months after infection.
Neurological and cognitive systems
Neurological and cognitive symptoms are a major feature of long COVID, including sensorimotor symptoms, memory loss, cognitive impairment, paresthesias, dizziness and balance problems, sensitivity to light and noise, loss of smell or taste and autonomic dysfunction, often impacting activities of daily living. Audiovestibular manifestations of long COVID include tinnitus, hearing loss, and vertigo.
Cognitive impairment is a characteristic that manifests itself independently of mental health conditions such as anxiety and depression. It occurs at similar rates in hospitalized and non-hospitalized patients.
Possible mechanisms of these neuropathologies include neuroinflammation, damage to blood vessels from coagulopathy and endothelial dysfunction, and damage to neurons.
In the eyes, loss of small corneal nerve fibers and increased dendritic cell density have been found in patients with long COVID, as well as significantly altered pupillary responses to light and altered retinal microcirculation.
Recent reports indicate low blood cortisol levels in long COVID patients compared to control individuals, with more than 1 year duration of symptoms. Low cortisol production by the adrenal gland should be compensated by an increase in adrenocorticotropic hormone (ACTH) production by the pituitary gland, but this was not the case, supporting hypothalamic-pituitary-axis dysfunction. adrenal. This may also reflect an underlying neuroinflammatory process.
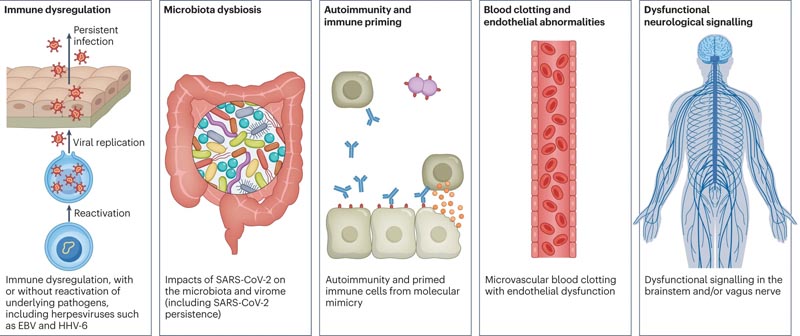
Figure 2. Hypothetical mechanisms of long COVID pathogenesis. There are several hypothesized mechanisms for long COVID pathogenesis, including immune dysregulation, microbiota disruption, autoimmunity, coagulation and endothelial abnormality, and dysfunctional neurological signaling. EBV, Epstein-Barr virus; HHV-6, human herpesvirus 6; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Myalgic encephalitis / Chronic Fatigue Syndrome (ME/CFS), dysautonomia and related conditions
ME/CFS is a multisystem neuroimmune disease that often appears after a viral or bacterial infection. The criteria include a "substantial reduction or impairment in the ability to participate at pre-illness levels of occupational, educational, social or personal activities" for at least 6 months, accompanied by profound fatigue that is not relieved by rest, along with post-exertional malaise, non-restorative sleep, and cognitive impairment or orthostatic intolerance (or both).
Many researchers have commented on the similarity between ME/CFS and long COVID. It is estimated that around half of people with long COVID meet the criteria for ME/CFS.
Abnormal findings consistent with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) include decreased natural killer cell function, T cell exhaustion and other T cell abnormalities, mitochondrial dysfunction, and vascular and endothelial abnormalities, including deformed red blood cells and volume. reduced blood.
Dysautonomia , particularly STOP, is commonly comorbid with ME/CFS and also often has a viral onset . STOP is associated with G protein-coupled adrenergic receptor and muscarinic acetylcholine receptor autoantibodies, platelet reserve deficiency, small fiber neuropathy, and other neuropathologies.
Reproductive system
Menstrual disturbances are more likely to occur in women and menstruating people with long COVID than in women and menstruating people without a history of COVID and who had COVID-19 but not long COVID.
Decreased ovarian reserve and reproductive endocrine disruption have been observed in people with COVID-19. Initial theories suggest that SARS-CoV-2 infection affects ovarian hormone production and/or endometrial response due to the abundance of ACE2 receptors in ovarian and endometrial tissue.
Research on myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) shows associations between ME/CFS and premenstrual dysphoric disorder, polycystic ovary syndrome, menstrual cycle abnormalities, ovarian cysts, early menopause, and endometriosis. Pregnancy, postpartum changes, perimenopause, and menstrual cycle fluctuations all affect ME/CFS and influence metabolic and immune system changes.
Viral persistence in penile tissue has been documented, as has an increased risk of erectile dysfunction, likely as a result of endothelial dysfunction. In one study, deficiencies in sperm count, semen volume, motility, sperm morphology, and sperm concentration were reported in individuals with long COVID compared to control individuals.
Respiratory system
Shortness of breath and cough are the most common respiratory symptoms and persisted for at least 7 months in 40% and 20% of long COVID patients, respectively. Several imaging studies involving non-hospitalized people with long COVID demonstrated lung abnormalities.
gastrointestinal system
Long COVID gastrointestinal symptoms include nausea, abdominal pain, loss of appetite, heartburn, and constipation. The composition of the gut microbiota is significantly altered in patients with COVID-19 and gut microbiota dysbiosis is also a key component of ME/CFS.
Most patients with long COVID symptoms and inflammatory bowel disease 7 months after infection had antigen persistence in the digestive mucosa. Higher levels of fungal translocation, from the intestinal and/or lung epithelium, have been found in the plasma of patients with long COVID compared to those without long COVID or SARS-CoV-2 negative controls, possibly inducing the production of cytokines.
Chronologies
The onset and time course of symptoms differ between individuals and depending on the type of symptom.
Neurological symptoms often have a delayed onset of weeks to months: among participants with cognitive symptoms, 43% reported a delayed onset at least 1 month after COVID-19 infection, and the delay was associated with older age. early. Several neurocognitive symptoms worsen over time and tend to persist, while gastrointestinal and respiratory symptoms are more likely to resolve.
Pain in the joints, bones, ears, neck and back are more common at one year than at 2 months, as are paresthesias, hair loss, blurred vision and swelling of the legs, hands and feet. Parosmia has an average onset of 3 months after the initial infection; Unlike other neurocognitive symptoms, it often decreases over time.
Few people with long COVID demonstrate a full recovery; one study found that 85% of patients who had symptoms 2 months after initial infection reported symptoms 1 year after symptom onset.
Long COVID in children
Similar to adults with long COVID, children with long COVID experience fatigue, post-exertional malaise, cognitive dysfunction, memory loss, headaches, orthostatic intolerance, difficulty sleeping, and difficulty breathing.
Liver injuries have been reported in children who were not hospitalized during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, and although rare, children who had COVID-19 are at increased risk for acute pulmonary embolism. , myocarditis and cardiomyopathy, venous thromboembolic events, acute and unspecified renal failure and type 1 diabetes.
Babies born to women who had COVID-19 during pregnancy were more likely to be diagnosed with neurodevelopmental disorders in the first year after birth. Experience from a pediatric long COVID center in treating patients suggests that adolescents with a moderate to severe form of long COVID have features consistent with myalgic encephalomyelitis/chronic fatigue syndrome.
Diagnostic tools and treatments
Although diagnostic tools exist for some components of long COVID, most are in development, including imaging to detect microclots, corneal microscopy to identify small fiber neuropathy, new fragmentation of the QRS complex on electrocardiograms as indicative of cardiac injury, and use of MRI. hyperpolarized magnetic resonance imaging to detect abnormalities in pulmonary gas exchange.
Early biomarker research suggests that levels of extracellular vesicles and/or immune markers indicating high cytotoxicity could be indicative of long COVID. Interestingly, dogs can identify people with long COVID based on sweat samples.
Although there are currently no broadly effective therapeutics for long COVID, treatments for certain components have been effective for subsets of populations.
Many ME/CFS strategies are effective for people with long COVID, including pacing and symptom-specific pharmacologic options (e.g., β-blockers for STOP, low-dose naltrexone for neuroinflammation, and intravenous immunoglobulin for immune dysfunction) and non-pharmacological options (including increasing salt intake for STOP, cognitive stimulation for cognitive dysfunction, and elimination diets for gastrointestinal symptoms).
It should be noted that exercise is harmful for long COVID patients who have ME/CFS or post-exertional malaise and should not be used as a treatment. One study of people with long COVID noted that physical activity worsened the condition for 75% of patients, and less than 1% experienced improvement.
Taken together, current options are based on small-scale pilot studies in long COVID or what has been effective in other diseases. Several additional trials are underway.
Impact of vaccines, variants and reinfections
The impact of vaccination on the incidence of long COVID differs between studies, in part due to different methods, time since vaccination, and definitions of long COVID.
One paper did not indicate any significant difference in the development of long COVID between vaccinated and unvaccinated people. Other studies indicate that vaccines provide partial protection, with a reduced risk of long COVID between 15% and 41%, with long COVID continuing to affect 9% of people with COVID-19.
Reinfections are becoming more common.
The impact of multiple instances of COVID-19, including the rate of long COVID in those who recovered from a first infection but developed long COVID after reinfection, and the impact of reinfection in those with pre-existing long COVID, is crucial to understand. to inform future policy decisions.
Challenges and recommendations
Testing problems
Most COVID-19 patients in the early waves did not have laboratory-confirmed infection, and PCR tests were difficult to access unless people were hospitalized. Probably only 1% to 3% of cases were detected through March 2020, and the CDC estimates that only 25% of cases in the US were reported between February 2020 and September 2021.
Although PCR tests are our best tool for detecting SARS-CoV-2 infections, their false negative rates remain high. Additionally, lack of access to testing, as well as false negative rates, have created a significant barrier to care, as many COVID clinics require PCR testing for admission.
Important errors
The narrative that COVID-19 only had respiratory sequelae led to a delayed understanding of the neurological, cardiovascular, and other multisystem impacts of COVID-19. Many clinics still disproportionately focus on respiratory rehabilitation, resulting in biased electronic health record data.
The narrative that initially mild cases of COVID-19, generally defined as not requiring hospitalization in the acute phase, would have no long-term consequences has also had subsequent effects on research. These so-called mild cases that result in long COVID often have different underlying biology than acute severe cases, but the same types of tests are used to evaluate patients.
General lack of post-viral knowledge and misinformation
The widespread lack of knowledge of viral-onset diseases, especially myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia, as well as often imperfect coding, prevents clinicians from identifying and documenting these conditions; This means that they are often absent from electronic health record data. Additionally, because research on ME/CFS and dysautonomia is not widely known or comprehensively taught in medical schools, long COVID research often does not build on previous findings and tends to repeat old hypotheses.
recommendations
Existing research is not enough to improve outcomes for people with long COVID.
To ensure an adequate response to the long-term crisis, we need research that builds on existing knowledge and includes patient experience, training and education of health and research staff, a public communication campaign, and strong policies and funding. to support research and care in long COVID.
Conclusions
|