| 1. Introduction |
Maternal mortality rates in the United States (US) remain the highest among developed countries and continue to rise. Cardiovascular diseases are the main etiology of maternal deaths in developed countries, accounting for more than a third of pregnancy-related deaths. The most important thing is that many are preventable.
Venous thromboembolism ( VTE), (i.e., deep vein thrombosis [DVT] and/or pulmonary embolism [PE]), is one of the leading cardiovascular etiologies of maternal morbidity and mortality, accounting for 9% of related deaths. with pregnancy. VTE at this stage can have serious short-term consequences as well as future complications such as post-thrombotic syndrome.
In this review, we aim to provide an overview of the epidemiology, mechanisms, risk factors, presentation and treatment of VTE during pregnancy and the postpartum period. Some potential preventive strategies are also highlighted. Another focus of this review is to explore knowledge gaps in the field and provide some directions for future research.
2. Epidemiology of VTE during pregnancy and the postpartum period
The risk of VTE among pregnant and postpartum women is 6 times higher compared to non-pregnant women. The risk also increases with gestational age, being approximately 2-fold higher during the first and second trimester and increasing up to 9-fold during the third trimester, while it is even higher during the postpartum period.
An analysis of the National Inpatient Sample (NIS) database (a US administrative database) that includes more than 50 million pregnancy and postpartum hospitalizations between 1998 and 2009, showed that PE rates increased by approximately 72% during admissions for delivery and 169% during postpartum hospitalizations. This increase was not solely attributed to the increased incidence of PE, but also to the more widespread use of computed tomography pulmonary angiography (CTPA) among pregnant and postpartum women.
In contrast, a recent NIS study of more than 37 million pregnancy and postpartum hospitalizations between 2007 and 2015 showed that rates of acute PE per 100,000 pregnancy-related hospitalizations did not change significantly during the study period (18.0 per 100,000 in 2007 compared to 19.4 per 100,000 in 2015).
3. Pathogenesis of VTE during pregnancy and the postpartum period
Pregnancy and the postpartum period are considered a prothrombotic state. This increased thrombogenicity occurs secondary to physiological changes and serves as an evolutionary protective mechanism against bleeding during childbirth. There is an activation of the coagulation cascade, including increased production of coagulation factors, decreased availability of free protein S, and decreased fibrinolytic factors, resulting in a hypercoagulable state.
In addition to hormone-mediated changes, there are other mechanisms that play a role in increasing the risk of thrombotic events during pregnancy and the postpartum period. These include increased venous pooling with resulting stasis, as well as mechanical obstruction by the uterus that could cause anatomical compression of the left iliac vein. There are also immunological changes, with increased cytokines and vascular endothelial dysfunction that may predispose to VTE ( Figure 1 ).
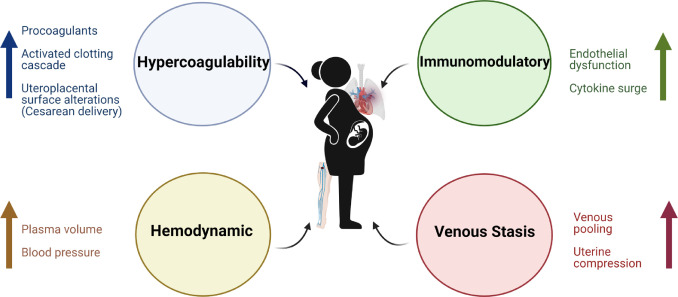
Figure 1. Pathophysiology of venous thromboembolism in pregnancy. Key pathophysiological mechanisms include immunomodulatory changes, hypercoagulability, hemodynamic changes, and venous stasis.
4. Risk factors
Aside from unique pregnancy-related mechanisms that predispose to VTE, risk factors could be broadly classified into pre-existing risk factors and pregnancy-specific risk factors.
4.1.Pre-existing risk factors
There are a number of pre-existing risk factors that independently increase the risk of VTE, including older age, obesity, previous VTE, thrombophilia, immobilization, recent travel, active cancer, and smoking.
4.2.Pregnancy-specific risk factors
4.2.1. Delivery by cesarean section
Caesarean section delivery is associated with an increased risk of VTE compared to vaginal delivery. It is also associated with an increased risk of PE compared to DVT. Compared with planned surgical delivery, emergency delivery confers a higher risk of VTE.
Cesarean delivery is associated with activation of the coagulation cascade, as well as alterations of the uteroplacental surface that increase the risk of thrombogenicity.
4.2.2.In vitro fertilization
In vitro fertilization (IVF) pregnancies are associated with an increased risk of VTE compared to normal pregnancies. Multiple IVF pregnancies were associated with higher rates of VTE than singleton IVF pregnancies.
The underlying pathogenesis of VTE in the setting of an IVF pregnancy has been linked to the estrogen surge. During IVF, controlled ovarian stimulation leads to multiple oocytes and supraphysiological levels of estrogen, resulting in a procoagulant state that increases the risk of VTE.
4.2.3.Preeclampsia
Preeclampsia is associated with an increased risk of VTE in the postpartum period. Although the mechanisms underlying this increased risk of thrombosis have not yet been fully elucidated, preeclampsia is related to altered expression of placental antiangiogenic factors that induce endothelial dysfunction, resulting in proteinuria and hypertension.
4.2.4.Infections
Infections are another recognized trigger of VTE during pregnancy and the postpartum period. There is activation of the coagulation cascade with platelet activation and aggregation, increased oxidative stress and deterioration of endothelial function, which ultimately increases the risk of thrombosis.
5. Clinical presentation and diagnosis
The symptoms and signs of VTE are often nonspecific and may overlap with the physiologic changes of pregnancy, including dyspnea, lower extremity edema, and tachycardia. Therefore, there is a possibility of misdiagnosing VTE during pregnancy and the postpartum period.
The diagnosis of symptomatic DVT is established by duplex ultrasonography (DUS) of the lower extremities, which is widely available and does not carry the risk of radiation to the fetus. The finding of DVT on DUS not only establishes the diagnosis of DVT, but also avoids the need for additional chest imaging if PE is clinically suspected, as the initial treatment would be the same. However, if PE is clinically suspected and DUS does not document DVT, additional diagnostic testing is required to evaluate PE through chest imaging using CTPA and/or lung perfusion scintigraphy (V/Q scan). ( Figure 2 ).
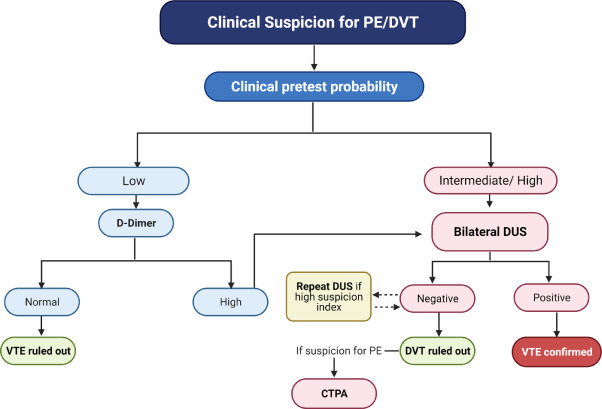
Figure 2. Diagnostic study for suspected acute venous thromboembolism in pregnancy. Algorithm that could be adopted during pregnancy and the postpartum period when there are signs and symptoms that raise suspicion of VTE, such as unilateral swelling of the extremities, dyspnea and/or hypoxia. VTE: venous thromboembolism; DVT: deep vein thrombosis; PE: pulmonary embolism; DUS: duplex ultrasound of the lower extremities; CTPA: computed tomography pulmonary angiography.
PD most commonly manifests as dyspnea. Importantly, two-thirds of pregnant and postpartum women have normal oxygen saturation at presentation, and therefore the absence of hypoxia should not rule out the diagnosis. In general, CTPA and V/Q scanning are two commonly used modalities for the detection/exclusion of PE in pregnant women. Although there has not been any direct comparison between CTPA and V/Q scanning, both have their advantages and disadvantages.
The emergence of more sophisticated modern imaging techniques has further reduced maternal and fetal exposure to radiation and therefore the risk associated with it. Chest MRI is another modality that can be used in the diagnostic armamentarium of PD, which has the advantage of being radiation-free; however, limited availability and unknown maternal and fetal safety data in humans are important limitations.
6. Management of VTE in pregnancy and postpartum
The mainstay of treatment for acute VTE in pregnancy and the postpartum period is anticoagulation. The anticoagulant of choice is heparin, preferably low molecular weight heparin (LMWH), although unfractionated heparin (UFH) can also be used, since both agents do not cross the placental barrier. This is in contrast to coumarin derivatives, such as warfarin, which cross the placenta and have the potential to cause teratogenicity, pregnancy loss, fetal bleeding, and neurodevelopmental deficiencies.
With progression of pregnancy, dosage adjustment may be necessary given changes in maternal weight to ensure adequate anticoagulation. Although pregnant women receiving LMWH often have subtherapeutic trough levels of anti-Xa and require higher doses to achieve target levels, routine monitoring of anti-Xa levels is not recommended given the predictable profile of LMWH.
Closer to delivery, twice-daily dosing of LMWH or preferably switching to UFH may be considered in view of the shorter half-life, reducing the risk of maternal hemorrhage and ensuring access to analgesia and neuraxial anesthesia. Although no studies have evaluated the optimal duration of anticoagulant therapy for the treatment of pregnancy-related VTE, anticoagulant therapy is recommended for the remainder of the gestational period and for at least 6 weeks after delivery and until at least 3 months of treatment in total.
6.1.Management of DVT
Although the mainstay of treatment for acute DVT is anticoagulation, the role of catheter-directed thrombolysis, which is a minimally invasive technique for the treatment of acute iliofemoral DVT, is not well established in pregnant women. The primary concern when performing catheter-directed thrombolysis is fetal exposure to radiation, particularly during the first trimester due to the high dose used.
However, in the second and third trimester, proper precautions, including protective and dose reduction techniques, could make this procedure safer. In cases of severe venous outflow obstruction after thrombolysis, iliac vein stenting could be delayed until after delivery, if possible.
6.2.Management of PE
Management of acute PE in pregnancy involves initial risk stratification and clinical evaluation, including hemodynamic status and right ventricular size and function, along with imaging, biomarker studies, and the use of validated scoring systems for stratify severity. Shared decision making through a multidisciplinary team of obstetrics, cardiology, pulmonology, hematology, vascular medicine, anesthesiology/intensive care, cardiothoracic surgery, and interventional radiology is important.
For low-risk acute PE, defined as hemodynamically stable with normal right ventricular function and absence of end-organ damage, the preferable choice is LMWH, while the other option is UFH. These patients can be managed on an outpatient basis, and do not require hospital admission. High-risk acute PE, characterized by hemodynamic instability with end-organ hypoperfusion, is rare during pregnancy but life-threatening and requires hospitalization. ( Figure 3 ).
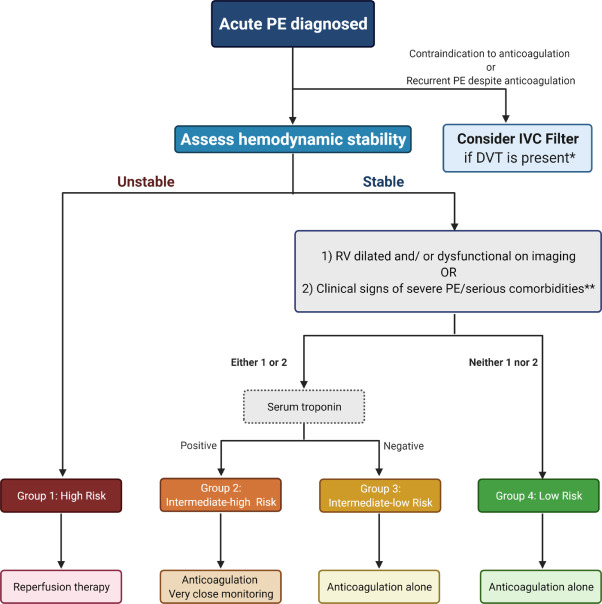
Figure 3. Management of acute pulmonary embolism in pregnancy. Demonstrates the scheme for early risk stratification and management of acute pulmonary embolism. PE: pulmonary embolism; IVC filter: inferior vena cava filter. *The IVC filter could be considered in women with pulmonary embolism without evidence of DVT, who are not candidates for systemic anticoagulation. * * History of malignancy, congestive heart failure, chronic interstitial or obstructive pulmonary disease.
For pregnant women with an absolute contraindication to anticoagulant treatment or with recurrent PE despite adequate anticoagulation, inferior vena cava (IVC) filters could be considered with the goal of preventing additional venous clots from reaching the pulmonary circulation. .
7. Recurrence and prevention of VTE in pregnancy and postpartum
Patients with pregnancy-associated VTE have a recurrence risk of up to 13% during subsequent pregnancies. Some evidence suggests that thromboprophylaxis is associated with a reduction in the risk of VTE recurrence.
Although drug therapy may reduce the incidence and recurrence of VTE during pregnancy and the postpartum period, universal thromboprophylaxis may not be a safe approach, due to the risk of maternal bleeding, as well as the risk of heparin-induced thrombocytopenia and osteoporotic fractures associated with heparinization. Therefore, routine thromboprophylaxis is recommended only for women considered at high risk for VTE based on certain factors, such as previous estrogen-associated VTE or inherited thrombophilia.
There have been no head-to-head studies comparing LMWH versus UFH in pregnant women, and data for prophylaxis come from the non-pregnant population. LMWH is generally administered during pregnancy at different doses (prophylactic, intermediate, therapeutic), and an evidence-based consensus regarding the optimal dosing strategy is lacking.
Pharmacological thromboprophylaxis is generally reserved for a selected population of pregnant women who are considered at high risk for VTE. A history of single idiopathic, pregnancy-associated, or estrogen-associated VTE is associated with a 10-fold increased risk and >1% absolute risk of VTE. Data suggest that women with a prior pregnancy-related or oral contraceptive-associated VTE are more likely to have a recurrent VTE during pregnancy than those with a prior unprovoked or non-hormone-associated VTE.
Recommendations for pharmacological thromboprophylaxis in specific thrombophilic disorders are subject to variability in different guidelines. In patients with hereditary thrombophilia, candidacy for thromboprophylaxis is determined by the type of hereditary thrombophilia, family history of VTE, and the antepartum versus postpartum period.
For women with antiphospholipid antibody syndrome and a history of three or more missed abortions, antepartum administration of prophylactic or intermediate-dose unfractionated heparin or prophylactic low-molecular-weight heparin combined with low-dose aspirin (75-100 mg) is recommended. / day).
Emergency cesarean delivery itself qualifies for postpartum prophylaxis in some guidelines, while others suggest pharmacological prophylaxis after cesarean section only if there are additional risk factors (such as obesity, advanced age, underlying malignancy, prolonged immobilization). Early ambulation and/or mechanical devices (eg, intermittent pneumatic compression) are suggested in those patients who undergo cesarean delivery and do not have any additional risk factors for VTE.
8. Future directions
VTE is a potentially preventable situation of maternal mortality. The morbidity and mortality associated with VTE remains alarming, calling for future efforts to fill the gaps that exist in our knowledge and understanding of the mechanisms, risk factors, and management.
First, existing VTE prediction scores are based on criteria that exclude pregnant women and are justified by characteristics that are rarely applied to pregnant women, such as advanced age or cancer. Therefore, there is a need for studies aimed at developing risk scores and predictive models that can be applicable to pregnant and postpartum women. There is also a need to develop educational programs to equip healthcare providers with knowledge to identify, manage, and prevent VTE in pregnant women.
Second, current recommendations for thromboprophylaxis during pregnancy and postpartum are stratified by thrombotic history or underlying thrombophilia. There are other important risk factors (e.g., age, race, BMI, infections, and pregnancy complications) that should be considered in the decision-making process regarding prevention of pregnancy-related VTE.
Third, further studies are recommended that validate diagnostic algorithms for VTE in pregnancy, using current radiological imaging techniques and low doses of radiation.
Finally, large-scale work is needed to evaluate the efficacy and safety of advanced therapeutic options for high-risk VTE; however, such studies can be difficult given the rarity of this condition. Addressing these knowledge gaps will help provide clinicians with a better understanding of the condition and help improve patient outcomes.
9. Conclusions VTE is one of the leading etiologies of maternal morbidity and mortality and is potentially preventable. There are pregnancy-mediated mechanisms that pose an increased risk of VTE in pregnant women compared to their non-pregnant counterparts, especially in the postpartum period. CTPA is the preferred diagnostic modality for suspected PE, especially with modern low-dose techniques that further reduce radiation exposure. While the treatment of DVT is primarily with anticoagulation, the treatment of PE depends on the risk stratification algorithm, ranging from anticoagulation in low-risk patients to advanced therapies in high-risk PE patients. There are some indications for thromboprophylaxis. Future studies are needed to fill some gaps in knowledge on this relevant topic. |
















