Conservative Management Versus Tonsillectomy in Adults With Recurrent Acute Tonsillitis in the UK (NATTINA): An Open-Label, Multicentre Randomized Controlled Trial
Summary
Background
Tonsillectomy is regularly performed in adults with acute tonsillitis, but with little evidence . A reduction in tonsillectomies has coincided with an increase in acute hospitalization of adults for complications of tonsillitis. Our objective was to evaluate the clinical effectiveness and cost-effectiveness of conservative treatment versus tonsillectomy in patients with recurrent acute tonsillitis .
Methods
This pragmatic, multicentre, open-label, randomized controlled trial was carried out in 27 UK hospitals. Participants were adults aged 16 years or older who were recently referred to secondary care otolaryngology clinics with recurrent acute tonsillitis.
Patients were randomly assigned (1:1) to receive tonsillectomy or conservative management using permuted random blocks of variable length. Stratification by recruitment center and baseline symptom severity was assessed using the Tonsil Outcome Inventory-14 (TOI) score (categories defined as mild 0-35, moderate 36-48, or severe 49-70).
Participants in the tonsillectomy group received elective surgery to dissect the palatine tonsils within 8 weeks of random assignment, and those in the conservative treatment group received standard nonsurgical care for 24 months.
The primary outcome was the number of sore throat days collected over 24 months after randomization, reported once a week with a text message. The primary analysis was performed in the intention-to-treat (ITT) population. This study is registered in the ISRCTN registry, 55284102.
Results
Between May 11, 2015 and April 30, 2018, 4165 participants with recurrent acute tonsillitis were assessed for eligibility and 3712 were excluded. 453 eligible participants were randomized (233 in the immediate tonsillectomy group vs. 220 in the immediate tonsillectomy group). in the conservative treatment group).
429 (95%) patients were included in the primary ITT analysis (224 vs 205). The median age of participants was 23 years (IQR 19-30), with 355 (78%) women and 97 (21%) men. The majority of participants were white (407 [90%]).
Participants in the immediate tonsillectomy group had fewer sore throat days over 24 months than those in the conservative treatment group (median 23 days [IQR 11–46] vs. 30 days [14–65]).
After adjusting for site and baseline severity, the incident rate ratio of total days with sore throat in the immediate tonsillectomy group (n=224) compared to the conservative treatment group (n=205) was 0.53 (95% CI: 0.43 to 0·65; <0·0001). 191 adverse events in 90 (39%) of 231 participants were considered related to tonsillectomy.
The most common adverse event was bleeding (54 events in 44 [19%] participants). No deaths occurred during the study.
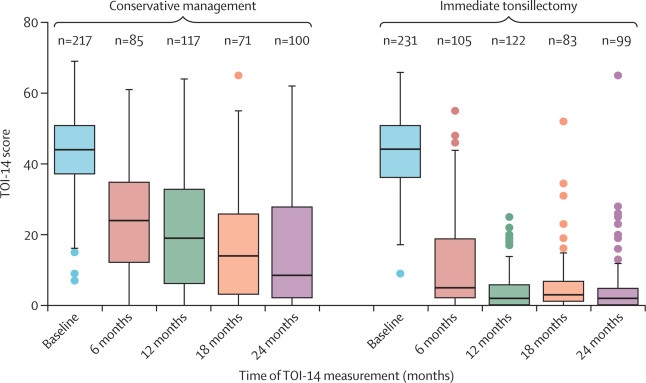
Tonsil Outcome Inventory-14 (TOI-14) Score at Baseline and at 6, 12, 18, and 24 Months The lines in the boxplot show the median scores and the boxes show the lower and upper quartiles. Mustaches are 1.5 times the IQR. The points are outliers outside that range. TOI-14=Tonsil Outcome Inventory-14.
Interpretation
Compared with conservative treatment, immediate tonsillectomy is clinically effective and cost-effective in adults with recurrent acute tonsillitis.
Comments
The largest study of its kind, carried out by the University of Newcastle, revealed that patients who had a tonsillectomy had 50% less sore throat over two years, compared to patients who did not have a tonsillectomy. Published in The Lancet , the study was funded by the National Institute for Health and Care Research (NIHR).
The experts also found that a tonsillectomy for those over 16 years of age was cost-effective compared to treatment with ad hoc painkillers and antibiotics.
Tonsillectomy Guarantee
A tonsillectomy is an operation that has been widely used to improve the quality of life of patients. However, in recent years, fewer adult tonsillectomies have been performed on NHS patients.
Dr James O’Hara, senior clinical lecturer at the University of Newcastle and consultant ear, nose and throat surgeon at Newcastle Upon Tyne Hospitals NHS Foundation Trust, said: "Tonsillectomy has been classed as an ’intervention of limited clinical value’. ’ due to the lack of studies supporting the operation.
“Over the last 20 years, the number of tonsillectomies performed in the UK has halved, while hospital admissions for complicated tonsillitis have more than doubled.
“There have been variations across the UK in primary care referrals for tonsillitis, with some patients having to experience three times the recommended number of episodes before being referred for tonsillectomy. “Our research should level the threshold for remission of this problem, and doctors can now be confident that tonsillectomy is effective for those suffering from recurrent tonsillitis.”
In the study, commissioned by the NIHR, almost 500 patients were randomized to early tonsillectomy or received treatment such as painkillers and antibiotics. Participants were only recruited if they met the current national guideline for offering tonsillectomy: seven episodes of tonsillitis in one year, five per year for two years, or three episodes for three years.
Those in the clinical trial who had the operation suffered half as many days with a sore throat over the next two years, including the two weeks of sore throat that followed the procedure.
Cost effective procedure
Research led by Newcastle also showed that it is more cost-effective for the NHS to offer tonsillectomy to eligible patients than to treat them with other conventional methods.
Dr O’Hara said: "While we now know that tonsillectomy is effective, patients still need to weigh the potential benefits in reducing long-term sore throat against 14 days of pain after the operation."
“There is also a risk that one in five patients will bleed after the operation and some will have to return to hospital. “More research is needed to improve the tonsillectomy operation to make it less painful and reduce the risk of bleeding.”
Professor Andrew Farmer, director of the NIHR Health Technology Assessment Programme, said: “These new results provide important findings suggesting that tonsillectomy benefits this group of patients compared to repeated courses of antibiotics and painkillers. “Once again, high-quality, independently funded research provides evidence that could improve health and social care practice and treatments.”
Newcastle University and Newcastle Hospitals are part of Newcastle Health Innovation Partners (NHIP). NHIP is one of eight prestigious Academic Health Sciences Centers (AHSC) across the UK, bringing together partners to deliver excellence in research, health education and patient care.
Patient case study
Secondary school teacher Elinor Barwick was the first patient recruited for the Newcastle-led trial, as her recurrent acute tonsillitis was affecting her life.
Elinor had suffered from terrible sore throats for many years, however things got significantly worse when she became a teacher as she needed to talk all the time, which left her in a lot of pain and discomfort.
Elinor’s tonsillitis often meant that it was difficult to swallow liquids and food. Additionally, her tonsillitis would leave her unable to speak as her throat would become extremely tight and swollen, and she would develop flu-like symptoms, all of which would affect her work and her quality of life. Elinor, from Gosforth, Newcastle, was recruited for the trial and was one of the participants who underwent the tonsillectomy procedure.
The mother of one of the patients said: “Recurring tonsillitis would affect my life as it would leave me in a lot of pain and at times make everyday life very difficult. “When I was asked to take part in the trial, I didn’t hesitate to say yes because it was being run by Newcastle University and would benefit patients like me.”
“Since having my tonsils removed, I have not had a day off work due to tonsillitis and my quality of life has greatly improved as I no longer suffer from severe sore throats.
“It is fantastic that research has been carried out in Newcastle into the importance of tonsillectomies in adults with acute recurrent tonsillitis to help patients like me in the future.”
Research in context Evidence before this study Iterations of the Cochrane review on the effectiveness of tonsillectomy in adults with recurrent tonsillitis identified low-quality evidence. Two small randomized trials with short follow-up suggested that the number of days with sore throat may be lower in the first 6 months after tonsillectomy than after conservative treatment. Given the limited evidence to support this common intervention, the UK’s National Institute for Health and Care Research commissioned a pragmatic clinical trial. In line with the recommendations of the Cochrane Review, the report specified that the primary outcome should be sore throat over 24 months. We searched MEDLINE for clinical trials published between January 1, 1996 and January 1, 2022, using the search term "tonsillectomy", limited to randomized trials and adults, and no further relevant clinical trials were found. Added value of this study To our knowledge, NATTINA is the largest randomized trial to evaluate the clinical efficacy and cost-effectiveness of surgical intervention in adults with sore throat. We show that, compared with conventional medical management, tonsillectomy is clinically effective and cost-effective. The incidence rate ratio for sore throat days was 0.53 with tonsillectomy over 24 months in the most conservative estimate and 0.42 when the actual treatment administered was taken into account. Implications of all available evidence NATTINA quantifies the comparative value of tonsillectomy for healthcare providers, optimizes the basis for shared decision making in adults with recurrent tonsillitis, and therefore has the potential to reduce regional variation in referrals for tonsillectomy. . But predominantly, NATTINA encourages timely access to care. |
Reference:
Conservative management versus tonsillectomy in adults with recurrent acute tonsillitis in the UK (NATTINA): a multicentre, open-label, randomized controlled trial. Janet Wilson et al. The Lancet .