Retatrutide, triple hormone receptor agonist for obesity: a phase 2 trial
Background
Retatrutide (LY3437943) is an agonist of glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1 , and glucagon receptors. Its dose-response relationships with respect to side effects, safety, and efficacy for the treatment of obesity are unknown.
Methods
We conducted a phase 2, double-blind, randomized, placebo-controlled trial in adults who had a body mass index (BMI, weight in kilograms divided by the square of height in meters) of 30 or more or who had a BMI 27 to less than 30 plus at least one weight-related condition.
Participants were randomly assigned in a 2:1:1:1:1:2:2 ratio to receive subcutaneous retatrutide (1 mg, 4 mg [starting dose, 2 mg], 4 mg [starting dose, 4 mg] , 8 mg [starting dose, 2 mg], 8 mg [starting dose, 4 mg], or 12 mg [starting dose, 2 mg]) or placebo once a week for 48 weeks.
The primary endpoint was percentage change in body weight from baseline to 24 weeks. Secondary endpoints included percent change in body weight from baseline to 48 weeks and weight loss of 5% or more, 10% or more, or 15% or more. Safety was also evaluated.
Results
We enrolled 338 adults , 51.8% of whom were men. The least squares mean percent change in body weight at 24 weeks in the retatrutide groups was −7.2% in the 1 mg group, −12.9% in the 4 mg combined group, −17.3 % in the 8 mg combination group, and -17.5% in the 12 mg group, compared to -1.6% in the placebo group.
At 48 weeks , the least squares mean percent change in the retatrutide groups was -8.7% in the 1 mg group, -17.1% in the 4 mg combined group, -22.8% in the combined 8 mg group and −24.2% in the 12 mg group, compared with −2.1% in the placebo group.
At 48 weeks, weight loss of 5% or more, 10% or more, and 15% or more had occurred in 92%, 75%, and 60%, respectively, of participants receiving 4 mg of retatrutide; 100%, 91% and 75% of those who received 8 mg; 100%, 93% and 83% of those who received 12 mg; and 27%, 9%, and 2% of those who received placebo.
The most common adverse events in the retatrutide groups were gastrointestinal; these events were dose-related, were mostly mild to moderate in severity, and were partially mitigated by a lower initial dose (2 mg vs. 4 mg). Dose-dependent increases in heart rate peaked at 24 weeks and decreased thereafter.
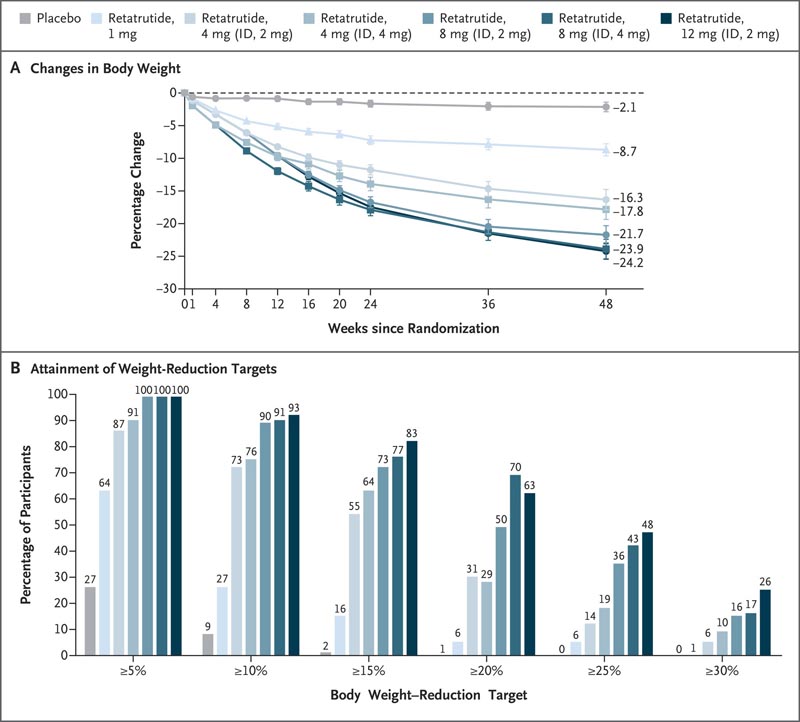
Figure: Changes in body weight with retatrutide compared to placebo . Panel A shows the percentage change in body weight from baseline to week 48, derived from a mixed model for repeated measures (MMRM) analysis for efficacy estimation. Values shown are least squares means; Bars indicate standard errors. Panel B shows the percentages of participants with percentage reductions in body weight of at least 5%, 10%, 15%, 20%, 25%, and 30% from baseline to week 48. Efficacy endpoints were analyzed with Data from all participants who underwent randomization, excluding those who discontinued treatment due to inadvertent enrollment. ID indicates the starting dose.
Conclusions In adults with obesity, treatment with retatrutide for 48 weeks produced substantial reductions in body weight. |
Reference : NEJM DOI: 10.1056/NEJMoa2301972
(Funded by Eli Lilly; ClinicalTrials.gov number, NCT04881760. opens in new tab.)
















