Highlights
|
Background
The role of glucocorticoids without surgical evacuation in the treatment of chronic subdural hematoma is unclear.
Methods
In this multicenter, open-label, controlled, noninferiority trial , we randomly assigned symptomatic patients with chronic subdural hematoma in a 1:1 ratio to a 19-day tapering course of dexamethasone or burr hole drainage.
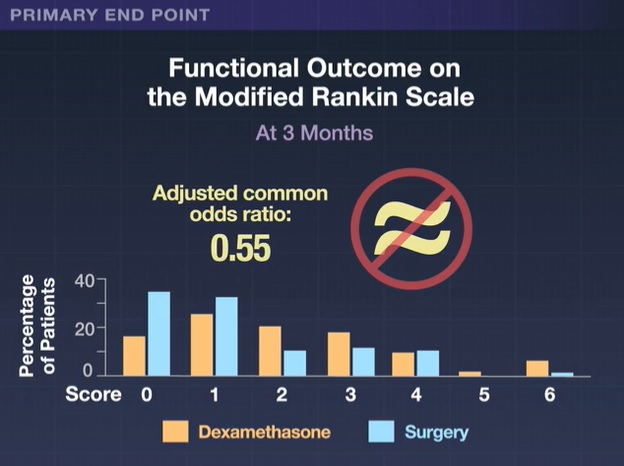
The primary endpoint was functional outcome at 3 months after randomization, assessed by the modified Rankin Scale score (range, 0 [no symptoms] to 6 [death]).
Noninferiority was defined by a lower limit of the 95% confidence interval of the odds ratio for a better functional outcome with dexamethasone than with surgery of 0.9 or more .
Secondary endpoints included scores on the Markwalder Symptom Severity Rating Scale and the Extended Glasgow Outcome Scale.
Results
From September 2016 to February 2021, we enrolled 252 patients from a planned sample size of 420; 127 were assigned to the dexamethasone group and 125 to the surgery group. The mean age of the patients was 74 years and 77% were men.
The data and safety monitoring board terminated the trial early due to concerns about safety and outcomes in the dexamethasone group.
The adjusted common odds ratio for a lower (better) modified Rankin Scale score at 3 months with dexamethasone than with surgery was 0.55 (95% confidence interval, 0.34 to 0.90). which did not demonstrate non-inferiority of dexamethasone. Scores on the Markwalder Rating Scale and the Extended Glasgow Outcome Scale generally supported the results of the primary analysis.
Conclusions
In a trial involving patients with chronic subdural hematoma that was discontinued early, dexamethasone treatment was found to be noninferior to trephination drainage with respect to functional outcomes and was associated with more complications and a higher likelihood of subsequent surgery.
(Funded by the Netherlands Organization for Health Research and Development and others; DECSA EudraCT number, 2015-001563-39).