Key points Is body mass index (BMI) or BMI change associated with gastrointestinal cancer risk? Findings This cohort study, using data from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, found that overweight and obese BMI in early and middle adulthood was associated with an increased risk of gastrointestinal cancer. Maintaining or increasing BMI while overweight or obese over time was also associated with an increased risk of gastrointestinal cancer. Meaning These findings suggest that overweight and obese BMI over time may increase the risk of gastrointestinal cancer. |
Colorectal cancer ( CRC) is the third most common cancer among men and women in the US. Although improvements in CRC detection and screening have changed the diagnosis of CRC to a more localized and regional disease, each year diagnoses a decreasing, but still staggering, number of incident cases of CRC. This may be due to a simultaneous increase in risk factors for the development of gastrointestinal (GI) cancer. Of particular interest, obesity rates are increasing globally. Obesity is associated with numerous negative outcomes, including the development of type 2 diabetes and other metabolic disorders; cardiovascular diseases, such as hypertension and stroke and cancer.
The World Cancer Research Fund and the International Agency for Research on Cancer have estimated that approximately 20% of cancers can be attributed to excess weight . Gastrointestinal cancers have been strongly associated with obesity, likely due to persistent chronic inflammation attributable to obesity. Chronic inflammation has been shown to be associated with an increased risk of several gastrointestinal cancers, including pancreatic (pancreatitis), esophageal (esophagitis and Barrett’s esophagus), and colorectal (ulcerative colitis and Crohn’s disease).
Epidemiological studies have consistently shown an increased risk of GI cancer among overweight and obese people. Additionally, an analysis of the Cancer Prevention Study II found that the risk of GI cancer-specific mortality increased from 1.86 to 4.52 among men with obesity and from 1.46 to 2.76 times among women with obesity compared to individuals with normal body mass index (BMI [calculated as weight in kilograms divided by height in meters squared]) (18.5-24.9 times increase).
Importance
In a population with significantly increasing rates of people who are overweight or obese, understanding the association of obesity with the risk of long-term diseases, such as cancer, is necessary to improve public health.
Aim
To investigate the association between body mass index (BMI) and risk of gastrointestinal (GI) cancer (colorectal cancer [CRC] and non-colorectal GI cancer) in the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) Screening Trial ).
Design, environment and participants
This retrospective cohort study was a secondary analysis of data from the PLCO cancer screening trial. Participants aged 55 to 74 years were enrolled and randomly assigned to the intervention group (screening group) or the control group at 10 screening centers between November 8, 1993 and July 2, 2001. The initial analysis of the PLCO cancer screening trial data occurred after 13 years of follow-up or December 31, 2009, whichever comes first.
Participants consented again in 2011 and continued follow-up or declined further follow-up. For those who re-consented, follow-up for incident cancers continued until December 31, 2014 or death, whichever came first. Data analysis for this secondary analysis was conducted from April 2022 to November 2022.
Exhibitions
Body mass index and aspirin use, defined as the frequency of use of aspirin or aspirin-containing substances in the past 12 months.
Main results and measures
Primary outcomes were diagnoses of colorectal cancer (CRC) and non-colorectal GI cancer. The association between BMI and cancer (CRC and non-colorectal GI cancer) was assessed using the Cox proportional hazards regression model. The association between cancer risk and change in BMI at different ages was further analyzed, and an exploratory analysis was performed to evaluate the risk of gastrointestinal cancer among aspirin users.
Results
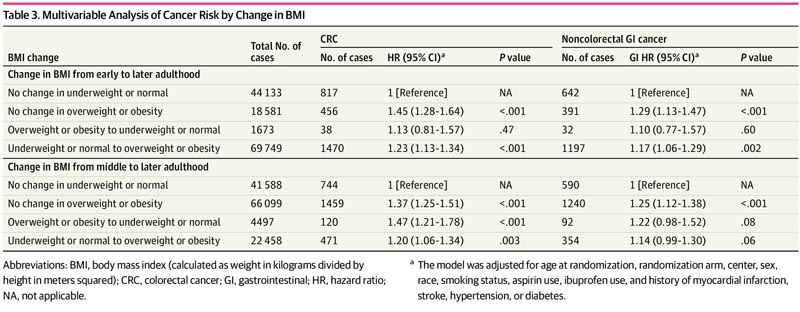
This analysis included 135,161 participants (median [range] age, 62 [55-78] years; 67,643 [50.0%] women). Overweight BMI in early adulthood (hazard ratio [HR], 1.23; 95% CI, 1.10-1.37) and overweight BMI in middle adulthood (HR, 1.23; 95% CI, 1.10-1.37). 95%, 1.13-1.34) and in late adulthood (HR, 1.21; 95% CI, 1.10-1.32), as well as obese BMI in middle adulthood (HR, 1.55; 95% CI, 1.38-1.75) and in late adulthood (HR, 1.39; 95% CI, 1.25-1.54) were associated with an increased risk of CRC.
Similar results were observed for the association with overall GI and non-CRC GI risk and BMI in middle and late adulthood.
Maintaining BMI overweight or obese or increasing BMI to overweight or obesity in adulthood was also associated with an increased risk of colorectal cancer (CRC). Using aspirin 3 or more times per week did not significantly modify this association.
Conclusions and relevance
In this secondary analysis of the PLCO Cancer Screening Trial, overweight and obese BMI in early and middle adulthood was associated with elevated risk of colorectal cancer (CRC) and non-colorectal gastrointestinal cancers.
The results of the current study prompt further exploration of the mechanistic role of obese BMI in carcinogenesis.
Discussion
In this cohort study, we found that overweight and obese BMI at different ages and change in BMI over time may be associated with an increased risk of gastrointestinal cancers.
Furthermore, we found that aspirin use 3 or more times per week did not modify this association.
The use of aspirin for cancer prevention has been well supported by decades of epidemiological evidence. Previous secondary analyzes demonstrated the effectiveness of aspirin in reducing CRC risk and bladder cancer mortality. However, the impact of BMI on this association has not been adequately delineated. Additionally, the US Preventive Services Task Force’s updated recommendations for the use of aspirin to prevent cardiovascular disease discuss the withdrawal of aspirin use for the prevention of CRC, which had previously been given a B grade for people ages 50 to 69 with a 10% or greater risk of cardiovascular disease, citing insufficient or conflicting evidence.
Obesity is the result of the accumulation and storage of white adipose tissue, or fat . Adipose cells can induce inflammatory response and promote immune cell dysfunction through the secretion of adipokines and proinflammatory cytokines, leading to further downstream mechanical dysregulation.
People with obesity are at increased risk of several conditions, including cancer. Interestingly, not all cancers are significantly associated with obesity; rather, it is more limited to those in which cancer cells grow close to fat cells, possibly due to the impact of fat cells on tumorigenesis. Research has indicated significant crosstalk between cancer cells and adipocytes. For example, coculture of CRC cell lines in vitro with adipocytes has demonstrated increased proliferation, migration, and transfer of nutrients (e.g., ketones and fatty acids) of cancer cells from adipocytes to cancer cells.
Transcriptomic analysis of the ColoCare Study, a prospective cohort of newly diagnosed CRC, found enrichment of pathways, such as fibrosis and glycolytic metabolism, associated with crosstalk between adipose tissue and tumor. Similar findings have been observed for non-colorectal gastrointestinal cancers. Although probably not the initiator, excess adipocytes promote tumorigenesis by supplying necessary nutrients to cancer cells and stimulating oncogenic pathways. Therefore, cancer prevention mechanisms that target the deleterious physiological effects of obesity may function to counteract tumorigenesis.
As found in the current study, obesity may alter the cancer preventive effect of aspirin.
Our results indicate that people with overweight and obese BMI had an increased risk of CRC and non-colorectal GI cancer with aspirin use 3 or more times per week, suggesting that aspirin may not be effective for prevention in states overweight or obese. Aspirin’s ability to protect against gastrointestinal cancers may be decreased in people with obesity due to inadequate dosing. One suggestion may be that people with obesity need to increase the frequency or dose of aspirin; However, increased aspirin use carries its own risks, such as gastrointestinal bleeding. In our analysis, we did not take into account participant dosing, a noted limitation of the study.
Additional studies evaluating the impact of aspirin dosage on cancer prevention, taking into account participants’ BMI or weight gain, are needed to better delineate the role of aspirin. The Cancer Prevention Project 3 (CaPP3) is currently underway to discern the effect of differential dosing of aspirin (100, 300, or 600 mg) in a cohort of people with Lynch syndrome. The CaPP3 study is ongoing; however, the eventual results of this study may be translated to the general average-risk population.
Final message In this secondary analysis of the PLCO Cancer Screening Trial , overweight and obese BMI in early and middle adulthood was associated with an elevated risk of colorectal cancer (CRC) and non-colorectal gastrointestinal cancers. The results of the present study prompt further exploration of the mechanistic role of obesity BMI in carcinogenesis. This association was not modified by aspirin use 3 or more times per week. The results of the current study prompt further exploration of the mechanistic role of obese BMI in carcinogenesis. Finally, future research should focus on identifying cancer prevention mechanisms for this high-risk group. |
















