Background
The risk of cardiovascular disease is increased among people with human immunodeficiency virus (HIV) infection, so data are needed on primary prevention strategies in this population.
Methods
In this phase 3 trial, we randomly assigned 7,769 HIV-infected participants at low to moderate risk of cardiovascular disease who were receiving antiretroviral therapy to receive daily pitavastatin calcium (at a dose of 4 mg) or placebo.
The primary outcome was the occurrence of a major adverse cardiovascular event, which was defined as a composite of cardiovascular death, myocardial infarction, hospitalization for unstable angina, stroke, transient ischemic attack, peripheral arterial ischemia, revascularization, or death from an undetermined cause. .
Results
The median age of participants was 50 years (interquartile range, 45 to 55); the median CD4 count was 621 cells per cubic millimeter (interquartile range, 448 to 827), and the HIV RNA value was below quantification in 5,250 of 5,997 participants (87.5%) with available data.
The trial was stopped early for efficacy reasons after a median follow-up of 5.1 years (interquartile range, 4.3 to 5.9).
The incidence of a major adverse cardiovascular event was 4.81 per 1,000 person-years in the pitavastatin group and 7.32 per 1,000 person-years in the placebo group (hazard ratio, 0.65; confidence interval [95% CI], 0.48 to 0.90; P = 0.002).
Muscle-related symptoms occurred in 91 participants (2.3%) in the pitavastatin group and 53 (1.4%) in the placebo group; Diabetes mellitus occurred in 206 participants (5.3%) and 155 (4.0%), respectively.
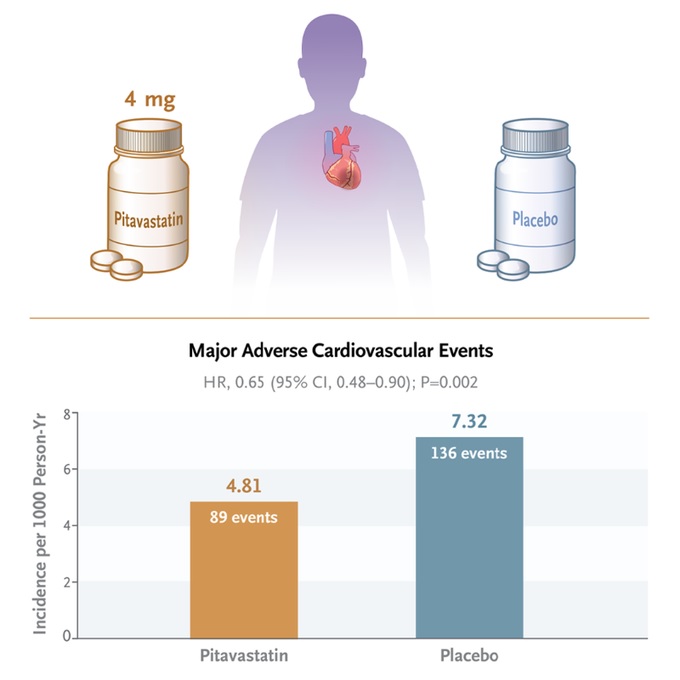
Conclusions HIV-infected participants who received pitavastatin had a lower risk of a major adverse cardiovascular event than those who received placebo during a median follow-up of 5.1 years. |
(Funded by the National Institutes of Health and others; REPRIEVE ClinicalTrials.gov number, NCT02344290. opens in new tab.)
















