Background
The role of catheter ablation in patients with symptomatic atrial fibrillation and end-stage heart failure is unknown.
Methods
We conducted a single-center, open-label trial in Germany involving patients with symptomatic atrial fibrillation and end-stage heart failure who were referred for heart transplant evaluation. Patients were assigned to receive catheter ablation and guideline-directed medical therapy or medical therapy alone.
The primary endpoint was a composite of death from any cause, left ventricular assist device implantation, or urgent heart transplantation.
Results
A total of 97 patients were assigned to the ablation group and 97 to the medical therapy group.
The data and safety monitoring board stopped the trial for efficacy reasons one year after randomization was completed.
Catheter ablation was performed in 81 of 97 patients (84%) in the ablation group and in 16 of 97 patients (16%) in the medical therapy group.
After a median follow-up of 18.0 months (interquartile range, 14.6 to 22.6), a primary endpoint event occurred in 8 patients (8%) in the ablation group and in 29 patients ( 30%) in the medical therapy group (hazard ratio, 0.24; 95% confidence interval [CI], 0.11 to 0.52; P < 0.001).
Death from any cause occurred in 6 patients (6%) in the ablation group and 19 patients (20%) in the medical therapy group (hazard ratio, 0.29; 95% CI, 0.12 to 0.72).
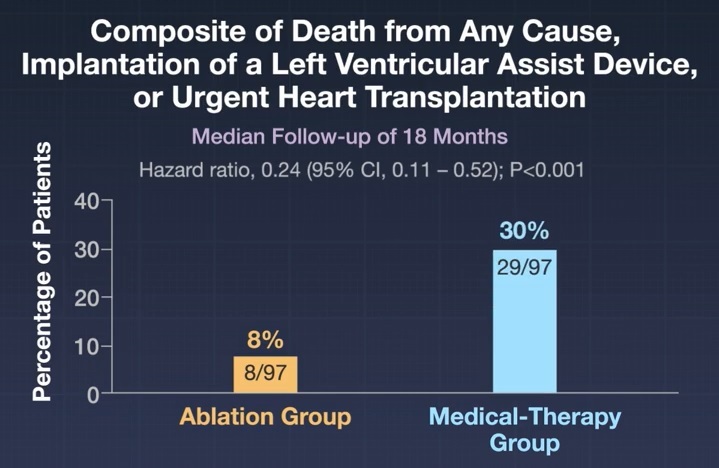
Conclusions Among patients with atrial fibrillation and end-stage heart failure, the combination of catheter ablation and guideline-directed medical therapy was associated with a lower likelihood of a combination of death from any cause, left ventricular assist device implantation, or heart transplantation. urgent than medical therapy alone. |
(Funded by Else Kröner-Fresenius-Stiftung; CASTLE-HTx ClinicalTrials.gov number, NCT04649801. opens in new tab.)
















