Highlights What is the clinical question being addressed? We investigated the risk of mild cognitive impairment following a diagnosis of AF in the United Kingdom. What is the main finding? Our study showed that atrial fibrillation (AF) was associated with a 45% increased risk of mild cognitive impairment and that cardiovascular risk factors and multi-comorbidity appear to be associated with this outcome. |
Mild cognitive impairment ( MCI) is an early stage of decline in cognitive function that is greater than that seen in healthy aging but of insufficient severity to meet the criteria for dementia. The causes of MCI are heterogeneous, and while MCI may be reversible in some individuals, it may reflect early pathological processes associated with dementia, with an annual conversion rate of ≈20%.
Factors associated with the development of or protection against mild cognitive impairment in patients with atrial fibrillation (AF) and subsequent development of dementia have not been fully elucidated.
We investigated the association of AF with mild cognitive impairment and subsequent dementia using data from routinely collected UK primary electronic health records (EHRs).
We used the UK-based linked electronic health record (EHR) of 4.3 million people between January 1, 1998 and May 31, 2016. All people with incident AF were included and the index date was defined as the date of the first recorded AF diagnosis.
For each case, we randomly selected 1 AF-free individual as a control from the study cohort who was matched to the sex and age at diagnosis of the individual with incident AF.
AF was defined as I48 of the International Statistical Classification of Diseases, 10th Revision (ICD-10) and corresponding terms from the Clinical Practice Research Datalink version 2 reading .
The primary outcome of the study was the incidence of mild cognitive impairment, defined as ICD-10 codes G31.8 and F06.7 and corresponding reading terms.
Follow-up ceased upon death, end of practice registration, cessation of data contribution to the CPRD, or end of the study period.
We studied the association between atrial fibrillation (AF) and mild cognitive impairment (MCI) in relevant subgroups, including age at AF diagnosis, sex, socioeconomic categories, stroke, and treatment with digoxin, anticoagulants oral and treatment with amiodarone.
We investigated the association between AF and MCI in the Cox proportional hazards model controlled for competing risk. Contradictory events (e.g., death) were treated as censored observations . Adjustment was made for age, sex, calendar year at study entry, socioeconomic status, smoking, hypertension, diabetes, obesity, hypercholesterolemia, hearing loss, thyroid disease, depression, atherosclerotic heart disease, peripheral arterial disease, heart failure , stroke, cancer, chronic kidney disease, liver disease and chronic obstructive pulmonary disease. We applied the same method to study the subsequent incidence of dementia in participants who developed MCI.
We analyzed data from 4,309,245 eligible people in the United Kingdom and identified 233,833 (5.4%) people with incident AF and a total of 233,747 without AF. The mean age was 74.2 years in both patients with AF and patients without AF.
During a median of 5.3 years of follow-up, there were a total of 4,269 incident cases of MCI in both AF and non-AF patients. People with AF had a higher risk of MCI than people without AF, with an adjusted HR of 1.45 (95% CI: 1.35-1.56).
In addition to AF, risk factors such as older age, female sex, greater socioeconomic deprivation, clinical history of depression, stroke, and multimorbidity were associated with an increased risk of MCI (hazard ratio ranging from 1 .08 (age in years) and 1.44 (history of depression at baseline), all P < 0.001).
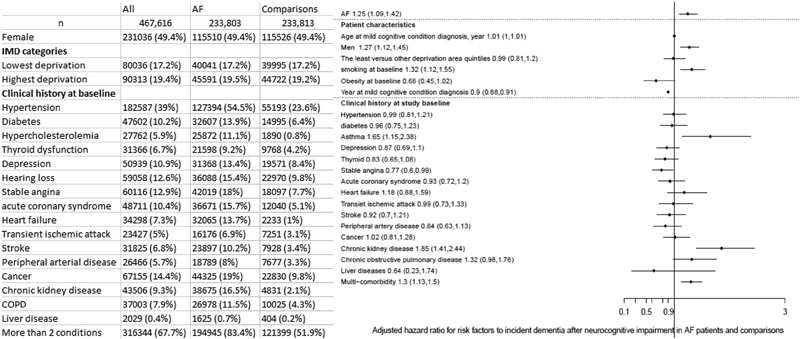
Figure 1 Study population and risk factors for incidence of dementia after a diagnosis of mild cognitive impairment. (Left) Characteristics of participants with AF and age- and sex-matched controls; (right) Adjusted HR associated with each risk factor for dementia incidence after MCI diagnosis. AF = atrial fibrillation; COPD = chronic obstructive pulmonary disease; IMD = Index of Multiple Deprivation.
Analyzes showed similar results in the population stratified by age at AF diagnosis, sex, socioeconomic deprivation, and history of stroke. Patients with incident AF who received digoxin treatment did not experience an increased risk of mild cognitive impairment (HR: 0.97, 95% CI: 0.53-1.78).
Similarly, while the risk of MCI was higher in patients with AF who did not receive treatment with oral anticoagulants and treatment with amiodarone, patients with AF who received treatment with oral anticoagulants and treatment with amiodarone had no risk of MCI.
Among people who developed MCI, there were 1,117 dementia diagnoses during or after the MCI diagnosis during the study period. People with AF were also associated with an increased risk of dementia among those who developed MCI (multiple-adjusted HR: 1.25 (95% CI: 1.09-1.42). Risk factors associated with risk risk of dementia were sex, smoking, asthma, chronic kidney disease, and multi-comorbidity.
Our study demonstrated that AF was associated with a 45% increased risk of MCI in a real-world, nationally representative cohort.
The results showed that age, greater sociodemographic deprivation, and a history of stroke were associated with an increased risk of MCI, but did not modify the association between FA and MCI. Both AF and MCI were frequently diagnosed in people older than 74 years when multi-comorbidity was present, and we found that diabetes, hypercholesterolemia, depression, and peripheral arterial disease are also associated with an elevated risk of MCI.
The progression from mild cognitive impairment to dementia appears to be, at least partially, mediated by cardiovascular risk factors and the presence of multiple comorbidities. Silent strokes are common in the AF population and have previously been associated with cognitive dysfunction.
Some limitations need to be acknowledged: As with all electronic health record studies, the potential lack of data granularity and level of detail in the data set is a potential limitation. It is also necessary to take into account the risk of unmeasured risk factors or comorbidities. We hope to have minimized this problem with a detailed characterization of the sample and the presentation of 16 frequently associated comorbidities. Underreporting of cognitive impairment, due to subtlety/nonspecificity of symptoms and suboptimal use of cognitive testing, is a problem for research in this field, also identified for EHR. 5 However, the ICD-10 has previously been used in studies of mild cognitive impairment with acceptable performance.
Finally, no increased risk of mild cognitive impairment was observed in people with AF receiving treatment with digoxin or amiodarone , with the risk in these patients being comparable to that of their peers without AF . However, the observational design of this study and the very wide confidence interval for these subgroups of patients (representing only 10%-20% of the AF sample) do not allow us to make strong inferences about causality, a possible role protective of these medications or unmeasured confounders.
Our findings emphasize the association of multicomorbidity and cardiovascular risk factors with the development of mild cognitive impairment due to AF and progression to dementia in the AF population. These data support the previous hypothesis of integrated AF care (combining anticoagulation, symptoms, and comorbidity management) as a way to prevent cognitive decline and progression to dementia, highlighting the need for a confirmatory clinical trial.
Footnotes
The study was approved by the Independent Scientific Advisory Committee of the Medicines and Healthcare products Regulatory Authority (UK) [18_228]. Dr Chung is supported by grants NIHR131227 and NIHR129463 from the National Institute of Health and Care Research (NIHR). Dr Providencia is supported by University College London British Heart Foundation Research Accelerator AA/18/6/34223 and NIHR grant NIHR129463. All other authors have reported that they have no relationships relevant to the content of this article to disclose.
















