Research published in Heart Rhythm finds that more than 10% of patients studied taking the antipsychotics quetiapine or haloperidol developed heart rhythm disorders
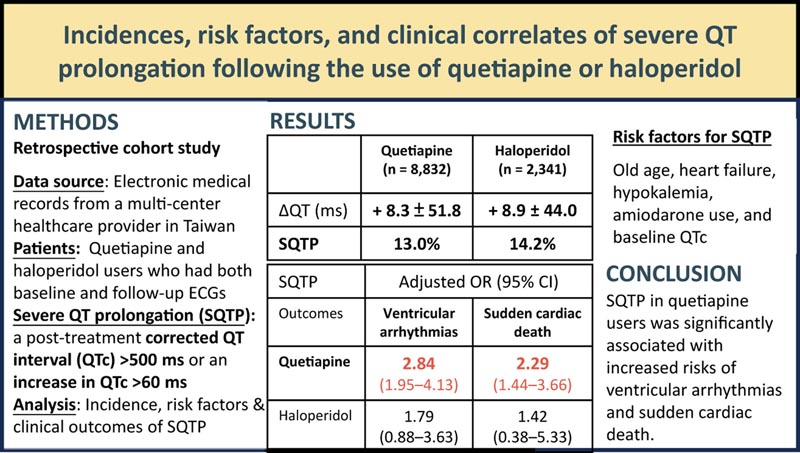
Summary Incidences, risk factors, and clinical correlates of severe QT prolongation after use of quetiapine or haloperidol Background Case reports suggest that use of quetiapine or haloperidol is associated with severe QT prolongation (QTPS) and torsades de pointes . Aim The purpose of this study was to examine the incidence, risk factors, and outcomes of severe QT prolongation (SQTP) in users of quetiapine and haloperidol. Methods This study accessed electronic medical records from a multicenter hospital healthcare system in Taiwan and included patients who received quetiapine or haloperidol therapy and underwent baseline and follow-up electrocardiograms. Severe QT prolongation ( SQTP) was defined as a post-treatment corrected QT interval (QTc) exceeding 500 ms or an increase in QTc interval of >60 ms compared to baseline. We analyzed the risk factors and outcomes of PQTS using multivariate logistic regression. Results Mean increases in QTc interval were +8.3 ± 51.8 and +8.9 ± 44.0 ms after administration of quetiapine (n = 8832) and haloperidol (n = 2341). Among these users, 1149 (13.0%) and 333 (14.2%) developed PTS, respectively. Common risk factors for PQTS included older age, heart failure, hypokalemia, amiodarone use, and baseline QTc interval. PTS in quetiapine users was significantly associated with ventricular arrhythmias (odds ratio 2.84; 95% confidence interval: 1.95–4.13) and sudden cardiac death (odds ratio 2.29; 95% confidence interval %: 1.44–3.66). Conclusion More than 10% of patients treated with quetiapine or haloperidol developed QTS, and many of them were exposed to risk factors for severe QT prolongation (SQTP). SQTP in quetiapine users was significantly associated with an increased risk of ventricular arrhythmias and sudden cardiac death. Clinicians should be alert for ventricular arrhythmias in quetiapine users who have risk factors for severe QT prolongation (SQTP). |
Comments
Use of the antipsychotic drugs quetiapine and haloperidol is associated with an increased risk of ventricular arrhythmias and sudden cardiac death (SCD) caused by drug-induced QT prolongation , reports a new study published in Heart Rhythm , a Heart Rhythm journal. Society, the Cardiac Electrophysiology Society and the Pediatric & Congenital Electrophysiology Society , published by Elsevier.
Caution is advised to monitor cardiac risks in patients prescribed these medications, the study authors and an accompanying editorial state.
The risks of heart disease associated with antipsychotic use have been a concern for the past 30 years. Previously, the drugs had been removed from the market or had their use restricted due to an unacceptably high risk of fatal ventricular arrhythmias. However, drug-induced cardiac arrhythmias remain an important clinical problem because there are drugs that increase the risk of sudden death, but they remain on the market because they fill an important clinical need and there are no safer alternatives.
Professor Jamie Vandenberg, PhD, MBBS, FHRS, of the Victor Chang Cardiac Research Institute, Darlinghurst, NSW, Australia, co-author of the editorial accompanying the study, explains: " Of the 41 drugs on the market in the United States that are in the list of known risks of heart rhythm disorders, five are antipsychotic drugs, the mainstay of treatment for schizophrenia and psychosis. The use of antipsychotic drugs is associated with an approximately two-fold increased risk of sudden cardiac death. If we cannot eliminate this risk then, at the very least, we should minimize the risk by identifying patients who are most at risk and treating them more closely.
The study’s principal investigator, Shang-Hung Chang, MD, PhD, of the Cardiovascular Division, Department of Internal Medicine, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan, adds: " The use of the antipsychotics quetiapine and haloperidol for treat Mental disorders are widespread. In an effort to improve patient safety and optimize treatment for people receiving these medications, we have investigated the incidences, risk factors, and clinical outcomes of severe QT prolongation for provide valuable information for healthcare professionals, patients and caregivers.
The research involved a retrospective analysis of electronic medical records from a large cohort of patients from a healthcare provider in Taiwan who received quetiapine or haloperidol therapy. The investigators evaluated the incidences, risk factors, and clinical correlates of severe QT prolongation (i.e., ventricular arrhythmias and sudden cardiac death) in these patients.
The most significant results of the study were that more than 10% of patients developed severe QT prolongation during follow-up and an increased risk of ventricular arrhythmias and sudden cardiac death in quetiapine or haloperidol users who developed severe QT prolongation.
Co-author Chun-Li Wang, MD, of the Cardiovascular Division, Department of Internal Medicine, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan, Taiwan, says the findings underscore the importance of closely monitoring patients receiving these medications. and implement appropriate risk mitigation strategies to ensure patient safety. "Physicians should be aware of the potential risks associated with the use of quetiapine, particularly the risk of severe QT prolongation and its associated outcomes, including ventricular arrhythmias and sudden cardiac death."
Professor Vandenberg comments: "It would be prudent to perform an ECG before and after starting an antipsychotic drug. If this is an option, one could stop the drug causing QT prolongation and try a different antipsychotic. But if this does not is practical, special attention should be paid to reducing other risk factors, such as prescribing other medications that may exacerbate QT prolongation and being alert for hypokalemia.
















