Reduction of Daily Maintenance Inhaled Corticosteroids in Patients With Severe Eosinophilic Asthma Treated With Benralizumab (SHAMAL): a Phase 4, Randomized, Multicenter, Open-Label Study
A landmark study has shown that severe asthma can be controlled by biological therapies, without the usual addition of high-dose inhaled steroids, which can have significant side effects.
Findings from the multinational SHAMAL study, published in The Lancet , showed that 92% of patients using the biological therapy benralizumab could safely reduce the dose of inhaled steroids and more than 60% could completely discontinue its use.
The study results could be transformative for patients with severe asthma by minimizing or eliminating the unpleasant, and often serious, side effects of inhaled steroids. These include osteoporosis, which increases the risk of fractures, diabetes and cataracts.
Asthma is one of the most common respiratory diseases worldwide (affecting almost 300 million people) and around 3 to 5% of them have severe asthma . This causes daily symptoms of shortness of breath, chest tightness and cough, along with repeated asthma attacks that require frequent hospitalization.
The SHAMAL study was led by Professor David Jackson, Director of the Severe Asthma Center at Guy’s and St Thomas’ and Professor of Respiratory Medicine at King’s College London.
Professor Jackson said: "Biological therapies such as benralizumab have revolutionized severe asthma care in many ways, and the results of this study show for the first time that steroid-related harms can be avoided in the majority of patients using this therapy."
Benralizumab is a biological therapy that reduces the number of inflammatory cells called eosinophils. It is produced in abnormal amounts in the airways of patients with severe asthma and is critically involved in the development of asthma attacks. Benralizumab is injected every four to eight weeks and is available in NHS specialist asthma centres.
The SHAMAL study was carried out at 22 sites in four countries: the United Kingdom, France, Italy and Germany.
The 208 patients were randomly assigned to taper off their high-dose inhaled steroids in varying amounts over 32 weeks, followed by a 16-week maintenance period. Approximately 90% of patients did not experience any worsening of asthma symptoms and remained exacerbation-free throughout the 48 weeks of the study.
Studies similar to SHAMAL will be necessary before firm recommendations can be made regarding the safety and effectiveness of reducing or eliminating the use of high doses of steroids with other biologic therapies.
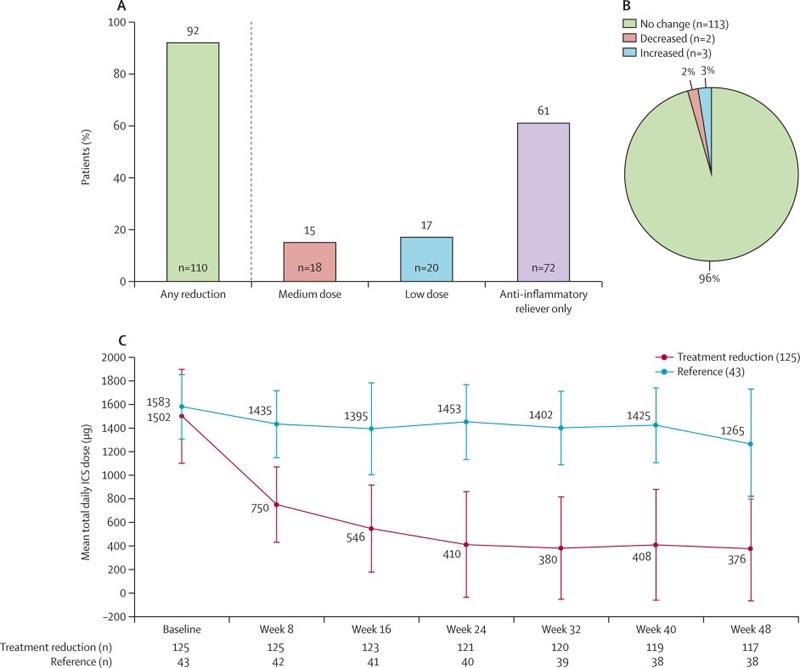
Figure : Reductions in ICS-formoterol maintenance dose throughout the study. (A) Patients reducing their maintenance dose of ICS-formoterol at the end of the taper period (week 32). (B) Patients who maintained their reduced dose throughout the maintenance period (week 48; n=118). (C) Changes in the mean total daily dose of ICS throughout the study. Proportions were calculated using the number of patients who did not miss a dose at week 32 and week 48 as the denominator. ICS = inhaled corticosteroids .
Research in context
Evidence before this study
We searched PubMed on August 10, 2023 for previous clinical studies evaluating the reduction of high-dose inhaled corticosteroid (IC) use in patients with severe asthma who respond well to biological treatment. The search terms “ICS reduction,” “biologic,” “benralizumab,” “safety,” “efficacy,” “severe asthma,” “severe eosinophilic asthma,” and “adverse effects” were used. No language or time restrictions were applied. The articles resulting from this search reported that biologics for the treatment of severe eosinophilic asthma have been shown to reduce asthma exacerbation rates and improve lung function. We also searched PubMed for reports of previous clinical trials of “dose reduction” studies of “inhaled corticosteroids” in “severe asthma,” without time or language restrictions. This search yielded two studies, only one of which evaluated a strategy to achieve ICS dose reductions. However, the results of that study showed that a biomarker-based corticosteroid titration strategy was not more effective than the control group approach. The Global Asthma Initiative recommendations suggest reducing ICS doses when possible in patients who respond positively to biologics. However, there is little clinical evidence on best practices regarding the safety and extent of inhaled corticosteroid (IC) dose reductions.
Added value of this study
SHAMAL was a phase 4, multicenter, randomized, open-label, active-controlled clinical trial that, to our knowledge, was the first to evaluate ICS reductions while maintaining asthma control in patients with severe eosinophilic asthma controlled with benralizumab. Almost all patients were able to reduce their inhaled corticosteroid (IC) dose while maintaining asthma control and remaining free of exacerbations. Patients in the reduction group used less than one-third of the total number of cumulative ICS doses compared to those in the reference group, with no change in asthma symptom control. Some patients withdrawing from regular inhaled corticosteroid (IC) use had a reduction in lung function, which was weakly correlated with a change in the fractional concentration of exhaled nitric oxide (FeNO), suggesting that a process mediated by Interleukin-13 could be the cause of impaired lung function.
Implications of all available evidence
These findings highlight the central role of eosinophils in driving exacerbations and poor symptom control in severe eosinophilic asthma . We demonstrate that patients controlled with benralizumab can safely minimize exposure to high doses of inhaled corticosteroids (ICs) and their associated adverse effects while maintaining disease control. Our findings underscore the opportunity to move away from high-dose inhaled corticosteroids (ICs) toward a precision medicine approach with improved outcomes in patients with severe eosinophilic asthma. Changes in FeNO concentration could further contribute to reductions in inhaled corticosteroid (IC) dosage by helping to identify patients at highest risk for deterioration in lung function.
The study was funded by AstaZeneca and carried out by researchers from renowned universities including Queens University Belfast, Université Paris-Saclay and Trinity College Dublin.















