Highlights
|
Summary
Psychosocial stress has a profound impact on well-being and health. The stress response is primarily associated with the amygdala, a crucial structure of the fear defense system, essential for social cognition and emotion regulation. Recent neuroimaging studies demonstrated how increased metabolic activity of the amygdala increases inflammation and leads to cardiometabolic disease.
The development of therapeutic strategies depends on our understanding of the factors that activate the fear defense system and the downstream molecular mechanisms that translate emotional stress into cellular damage. Fear of emotions as a consequence of attachment trauma is the most important trigger of the maladaptive activation of the fear defense system. The central molecular pathways are enhanced myelopoiesis and upregulated proinflammatory gene expression, glucocorticoid and insulin resistance, and oxidative stress.
Therapeutic strategies can benefit from holistic approaches. Psychotherapy can reduce maladaptively increased activation of the fear defense system. Biological interventions can buffer the detrimental effects of oxidative stress on the body.
It has become commonplace that mental stress is detrimental to health and it is known that people with mental disorders have an increased risk of developing cardiometabolic diseases and a significantly reduced life expectancy. This review will show that mental stress is caused by the maladaptive activation of the fear defense system and will highlight the role of emotions, which are the main innate motivation and regulation system in humans, in this process.
- First, we give an overview of the fear defense system and the central role of the amygdala .
- Second, we describe how activation of the fear defense system leads to inflammation .
- Third, we demonstrate the most important triggers of the fear defense system.
- Fourth, we describe the pathways from amygdala activation to oxidative stress .
- Fifth, we map the clinical and social implications.
The amygdala and the fear defense system
The fear defense system is an innate system that organizes species-typical defensive responses hardwired to respond to threats that promote survival. Activation of defensive behavior begins with an arousal reaction processed by the amygdala that occurs without conscious awareness. The conscious perception of this reaction is the feeling of anxiety (for example, muscle tension in the neck, sweating, increased heart rate, hyperventilation, vasospasm with cold hands).
The patterns of defensive responses can be classified as follows:
Fight or flight represent active responses (in humans, for example, getting angry and talking or becoming submissive).
Freezing is a state of attentive immobility, which allows the mammal to scan the environment and prepare fight or flight reactions (e.g., enhanced vigilance state with activated tense body). In situations of inescapable threat, mammals react with tonic immobility . This terminal defense has the function of deactivating the predator’s killer reflex when the mammal has been caught.
In humans, this defense is characterized by experiences of numbness, fear, perceptual distortions such as derealization and depersonalization, and hopelessness. A similar defense response is collapsed immobility (in humans, for example, fear-induced fainting due to cerebral hypoxia).
The final response is inactive immobility , which occurs after periods of acute stress when the mammal has returned to a safe environment and serves to recover. This defensive response is the underlying brain mechanism of clinical conditions such as chronic pain syndromes or prolonged exhaustion .
These defense reactions have specific neurohumoral pathways that include the amygdala, hypothalamus, periaqueductal gray, and the sympathetic and vagal nuclei.
Maladaptation of the fear defense system and dysregulated anxiety constitute the psychophysiological bases of common mental disorders.
Triggers of the fear defense system and the role of emotions
Innate stimuli or external threats can trigger the fear defense system. In humans, for example, exposure to noise or aversive auditory stimuli represents a natural stimulus that activates the amygdala.
Exposure to psychosocial stressors, such as poor and low-income residential areas, are pervasive external threats that activate the fear defense system with deleterious effects on health and survival.
However, in humans, experience-dependent triggers are of particular importance for the activation of the fear defense system. The underlying mechanism is called Pavlovian defense or fear conditioning : insignificant stimuli become threat signals when they occur alongside biologically significant threats.
In humans, more than other species, brain development is prolonged to allow optimal adaptation through the acquisition of complex behaviors. Attachment experiences with parents play a critical role in the acquisition of complex cognitive and affective behaviors and play a unique role in fear conditioning.
Therefore, early caregiver adversities such as abuse (physical, emotional, sexual) or neglect of the baby’s emotional needs (for example, due to parental mental disorders or early losses of caregivers or adversities social) are very powerful stressors for neurological development.
These effects are particularly processed by the amygdala and medial prefrontal cortex (mPFC). The mPFC is a brain structure important for social cognition and the regulation of emotions and behavior.
During human development, the amygdala and mPFC are forming rich interconnections. Indeed, secure attachment experiences during infancy are associated with more adaptive maturation of amygdala-mPFC connectivity and smaller amygdala volumes compared to insecure attachment experiences.
Attachment trauma predicts greater amygdala volume in adulthood and results in greater amygdala response to salient stimuli. In this context, it is essential to recognize that the attachment relationship between babies and parents is regulated by basic emotions, such as happiness, sadness, anger, disgust, surprise and fear.
Emotions are the main motivation system for humans.
Babies, unable to speak, communicate with their parents through the expression of their feelings. Emotions are intra- and interpersonal regulators. Anger , for example, initiates types of self-affirming behavior of the baby toward the parent . Parents’ adaptive responses toward the angry infant will increase the infant’s self-efficacy and trust in the attachment figure.
However, the parent’s reaction can also result in fear conditioning of the emotion "anger" . Imagine that the parent reacts with anxiety or frightens the infant by becoming aggressive or withdrawing, then the emotion of anger becomes a threat signal to the infant.
Due to the immense dependence on the attachment figure, the baby’s most crucial motive is to maintain the bond with the father and avoid any behavior that may jeopardize the bond with the father. Mental disorders are the result of such learned affective phobias and the avoidance or defense of such emotions in adulthood.
As emotions are the basic motivational system of humans, conditioned fear of emotions and defense against feelings has a profound impact on identity development, self-regulation, and interpersonal skills.
A crucial marker of mental health, therefore, is the ability to experience and express the full range of emotions adaptively.
It is important to note that a lot of unhealthy behavior, for example smoking, is a way of coping with maladaptation by activating the fear defense system.
Activation and inflammation of the tonsil
Recent imaging studies demonstrated, for the first time, how activation of the amygdala-based fear defense system leads to somatic diseases.
In the first study, Tawakol et al. (2017) demonstrated using 18F-fluorodeoxyglucose positron emission tomography that increased metabolic activity of the amygdala predicted the independent and robust development of atherosclerosis and cardiovascular disease events .
Amygdalar metabolic activity was further correlated with self-reported stress level, and perceived stress was associated with measures of inflammation. Increased tonsillar metabolic activity induced through sympathetic nervous system pathways, activation of the bone marrow and therefore increased release of inflammatory cells as a consequence of increased vascular inflammation.
The same pathways were elucidated in a sample of patients with psoriasis , a chronic inflammatory skin disease: increased metabolic activity of the amygdala led to activation of the hematopoietic system with increased release of activated monocytes that stimulate inflammation and atherosclerosis.
Other neuroimaging studies showed that amygdala activity was associated with basal visceral adiposity , as well as with an increase in visceral adiposity and the development of adiposity-independent diabetes mellitus . Again, these detrimental health effects were primarily mediated by an increase in proinflammatory leukopoiesis induced by activation of the fear defense system.
Amygdala activation and oxidative stress
The neurochemical cascade induced by maladaptive activation of the amygdala-related fear defense system may have long-lasting consequences such as inflammation, atherosclerosis, changes in insulin sensitivity, and cardiovascular diseases. Chronic activation of the amygdala leads to activation of the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis (Fig. 1).
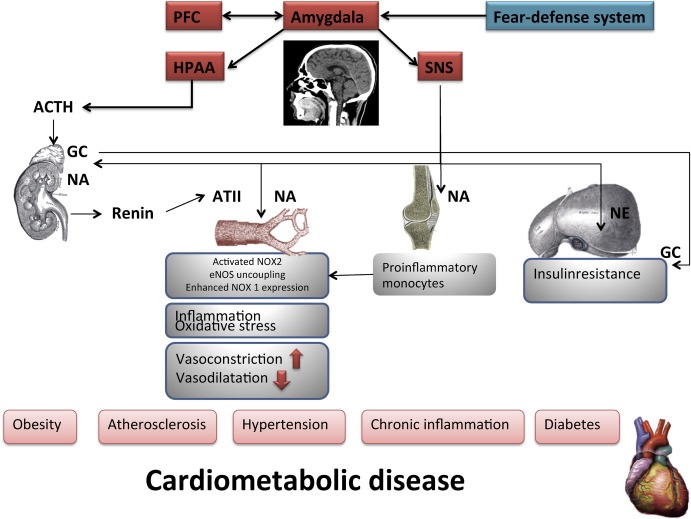 Figure 1. Chronic activation of the amygdala leads to activation of the hypothalamic-pituitary-adrenal (HPA) axis, as well as activation of the sympathetic nervous system (SNS). Activation of SNS leads to renin secretion and release of angiotensin II (ATII). ATII activates NOX2 (NADPH oxidase 2) in endothelial cells, resulting in oxidative stress. This can lead to uncoupling of endothelial nitric oxide synthetase (eNOS). Oxidative stress in endothelial cells activates NF-kB (nuclear factor k-light chain enhancer of activated B cells), leading to the induction of adhesion molecules leading to vascular inflammation. The HPA axis is mediated by CRF (corticotropin-releasing factor), ACTH (adrenocorticotropic hormone), and corticosteroids. When stimulated, the HPA axis rapidly releases glucocorticoids (GCs). GC enhances NOX1 (NADPH oxidase 1) expression in vascular muscle cells. GC and NA (Norepinephrine) can lead to decreased insulin sensitivity. The scheme was partly adopted from Li et al. Br J Pharmacol. 2019 (Li, Kigallen and Münzel, 2019).
Figure 1. Chronic activation of the amygdala leads to activation of the hypothalamic-pituitary-adrenal (HPA) axis, as well as activation of the sympathetic nervous system (SNS). Activation of SNS leads to renin secretion and release of angiotensin II (ATII). ATII activates NOX2 (NADPH oxidase 2) in endothelial cells, resulting in oxidative stress. This can lead to uncoupling of endothelial nitric oxide synthetase (eNOS). Oxidative stress in endothelial cells activates NF-kB (nuclear factor k-light chain enhancer of activated B cells), leading to the induction of adhesion molecules leading to vascular inflammation. The HPA axis is mediated by CRF (corticotropin-releasing factor), ACTH (adrenocorticotropic hormone), and corticosteroids. When stimulated, the HPA axis rapidly releases glucocorticoids (GCs). GC enhances NOX1 (NADPH oxidase 1) expression in vascular muscle cells. GC and NA (Norepinephrine) can lead to decreased insulin sensitivity. The scheme was partly adopted from Li et al. Br J Pharmacol. 2019 (Li, Kigallen and Münzel, 2019).
The sympathetic nervous system, oxidative stress and proinflammatory monocytes
Activation of the SNS in the rapid release of adrenaline and norepinephrine, mainly by the adrenal medulla. The SNS stimulates renin secretion and the production of angiotensin II (ATII). NADPH oxidase (NOX2) in endothelial cells is activated by ATII, resulting in oxidative stress.
The term oxidative stress is commonly defined as an excess of pro-oxidative factors, reactive oxygen species (ROS) and reactive nitrogen species (RNS) over antioxidants. High concentrations of ROS and RNS, and low antioxidant capacity, can damage various cellular components. The consequence is severe cellular distress with impaired cellular function and cell death.
Activated NOX2 can induce uncoupling of endothelial nitric oxidase (eNOS). Uncoupling of eNOS leads to reduced NO production. Furthermore, norepinephrine enhances NOX expression and promotes the adhesion of immune cells to the vascular wall. Infiltration of immune cells causes vascular oxidative stress through NOX2 activity.
Furthermore, ROS signaling activates transcription factors, leading to the expression of several genes involved in tumor suppressive and antioxidant action. For example, ROS signaling can enhance the expression of nuclear factor-kappa B (NF-κB) [33]. NF-κB regulates the expression of almost 500 different genes, including enzymes, for example, inducible NO synthase (iNOS), cytokines and tumor necrosis factor (TNF) [59].
NF-κB can be activated transiently by various stimuli, such as acute exposure to alcohol, cigarette smoke, physiological stress, but also by mental stress that produces neuroinflammatory responses in mice [1,69], which represents a "stress sensor" .
SNS activation enhances monocytopoiesis in the bone marrow, resulting in the expansion of pro-inflammatory monocytes . Furthermore, chronic inflammation leads to a change in hematopoietic topography from the bone marrow to the spleen. The migration of hematopoietic progenitor cells from the bone marrow to the periphery contributes to increased leukocyte production.
Accumulating data suggest that psychosocial stress and an unhealthy lifestyle initiate the displacement of hematopoietic stem cells and the release of progenitors from the bone marrow to the periphery.
Furthermore, increased sympathetic nervous system activity decreased CXC chemokine 12 (CXCL12) expression in the hematopoietic stem cell niche and enhanced neutrophil and monocyte production in stress-exposed mice. This led to extensive release of inflammatory leukocytes into the circulation and promoted atherosclerotic plaque inflammation.
The HPA axis and glucocorticoids
The HPA axis cascade is highly effective in maintaining allostasis and adaptation to stressful stimuli.
In depression , HPA axis activity is associated with hypercortisolemia and reduced inhibitory feedback. In solitary individuals , activation of the HPA axis is a constant finding. The HPA axis is mediated by corticotropin releasing factor (CRF), adrenocorticotropic hormone (ACTH). When stimulated, the HPA axis rapidly releases high concentrations of glucocorticoid stress hormones, resulting in increased cellular metabolism and the spontaneous formation of oxygen and nitrogen radicals.
Glucocorticoid release follows the circadian rhythm , with the highest levels in the morning and the lowest levels in the evening. Glucocorticoids govern physiological function, including immunity, insulin sensitivity , cardiovascular activity, reproductive processes, neurodegeneration, and apoptosis.
Long-term maintenance of a maladaptive defensive state can lead to hypersecretion of glucocorticoids and dysregulation of glucocorticoid receptor (GR) function, including degradation of GR, disruption of GR translocation, binding of GR- DNA and changes in the phosphorylation state of GR. Previous findings suggest that glucocorticoid receptors can translocate into mitochondria and modulate mitochondrial gene expression.
Resistance to glucocorticoids can be potentiated by inflammatory cytokines. Regulation of mitochondrial function by corticosterone is associated with neuroprotection . Treatment with low doses of corticosterone had a neuroprotective effect. Treatment with high doses of corticosterone was toxic to cortical neurons. The regulation of neuronal mitochondrial function by steroids is also related to neuroprotection and synaptic plasticity.
The release of endogenous CRF can be measured in the amygdala during stress. Potent anxiolytic actions are observed when CRF receptor antagonists are administered to the amygdala. CRF-containing amygdala neurons can be directly modulated by alterations in circulating glucocorticoids through glucocorticoid receptors, which are expressed in amygdaloid CRF-containing neurons.
Mental disorders are associated with oxidative stress
Mental disorders are associated with increased inflammation.
This relationship has been demonstrated for anxiety disorders (post-traumatic stress disorder, generalized anxiety disorder, panic disorder, and phobic disorders), somatic symptom disorders, and particularly for major depression . In depressed patients, inflammation is associated with neurochemical, neuroendocrine, and behavioral changes.
Inflammatory processes increase the production of ROS and RNS and oxidative stress in both the periphery and the central nervous system. Depressive disorders are associated with biomarkers of increased oxidative stress. Oxidative stress causes premature aging, as reflected by telomere shortening in patients with major depression, and plays a role in the onset and course of depression.
NOX2, as an essential source of oxidative stress, has been observed to be associated with severe life stress. Furthermore, there is a negative correlation between depression and antioxidant status. Antidepressant-like effects can be induced by reducing NO levels or blocking NO synthesis in the brain.
In patients with major depressive disorder, long-term treatment with antidepressant drugs had positive effects on oxidative damage and inflammatory profile, as well as on the activities of antioxidant enzymes.
Psychotherapy can also modulate oxidative stress in patients with major depression . The treatment reduced basal levels of increased serum NO to values close to the healthy control group. Additionally, psychotherapy, through affective labeling (expressing feelings in words), can reduce anxiety.
Taken together, chronic activation of the fear defense system leads to activation of SNS and HPA axis. This leads to uncoupling of eNOS, changes in GC sensitivity and increased monocytopoiesis in the bone marrow, chronic inflammation and related diseases (atherosclerosis, obesity, diabetes).
Clinical and social implications
At the population level, actions to overcome social disparities and increase healthy and safe environments would represent measures to reduce anxiety, inflammation and oxidative stress. Other population-based approaches include legislative measures to promote antioxidant nutrition and a physically active lifestyle as recommended by recent guidelines.
At the individual level, pharmacological interventions may be potentially helpful.
|
There are many evidence-based interventions to improve the adaptability of the fear defense system, improve emotional health and improve lifestyle, ranging from intensive mental health care, psychotherapy to mindfulness meditation to improve self-care and relaxation. .
















