Current evidence suggests that COVID-19 severity and mortality are higher in men than women, while women may be at higher risk of COVID-19 reinfection and development of long COVID.
Differences between sexes have been observed in other infectious diseases and in response to vaccines. Sex-specific expression patterns of proteins mediating virus attachment and entry, and divergent reactions of the immune and endocrine system, particularly the hypothalamic-pituitary-adrenal axis, in response to acute stress could explain the increased severity. of COVID-19 in men.
Sex hormones, comorbidities, and sex chromosome complement influence these mechanisms in the context of COVID-19.
Due to its role in the severity and progression of SARS-CoV-2 infections, we argue that sexual dimorphism has potential implications for disease treatment, public health measures, and monitoring of patients predisposed to the development of long COVID. .
We suggest that sex differences could be considered in future pandemic surveillance and treatment of COVID-19 patients to help achieve better disease stratification and better outcomes.
Sex differences in COVID-19
Women are less affected by insulin resistance, have fewer cardiovascular risk factors, and have more favorable protein, microbiome, lipidome, and microRNA expression profiles than men.
Since insulin resistance and altered glucose metabolism are key risk factors for developing severe COVID-19, women could have a more advantageous metabolism, preventing disease progression.
Greater predisposition to inflammation in men
Infections with a variety of pathogens are associated with different immune responses and disease outcomes depending on sex. Men are more likely to have a less potent immune response and therefore greater susceptibility or vulnerability to infections.
Obesity has previously been described as a predictor of the severe course of COVID-19.34 However, beyond BMI, the distribution of fat deposits also appears to be important ; Visceral fat, which accumulates more in men than women, has been shown to be associated with more severe COVID-19.
Furthermore, adipose tissue in men contains more macrophages and immune cells with higher and longer cytokine concentrations than in women. This could become the source of more rapid and intense systemic inflammation in men, contributing to the detrimental increase in cytokines (cytokine storm) seen in severe SARS-CoV-2 infections.
Sexual dimorphism in the response to adrenal stress and COVID-19
The hypothalamic-pituitary-adrenal (HPA) axis, responsible for integrating and managing the body’s internal and external stress stimuli, demonstrates clearly sex-biased activity, with striking sex differences in the neuroendocrine response, particularly to acute stress.
Women generally present with increased glucocorticoid secretion in response to various acute stressors.
Adult sex differences in the neuroendocrine response to acute stress are partly the result of interactions between the HPA axis and the endocrine system, which controls reproduction.
Therefore, by increasing dihydrotestosterone or estradiol production, the hypothalamic-pituitary-gonadal axis modulates HPA axis function in adults in a sex-dependent manner. Estradiol treatment increases HPA axis activity, but endogenous estrogens have also been reported to have inhibitory effects.
The importance of the HPA axis, and particularly the adrenal glands, in the context of COVID-19 is supported by recent findings demonstrating that the adrenal glands are a potential target for SARS-CoV-2 infection; The resulting cellular damage could potentially predispose COVID-19 patients to adrenal dysfunction .
More robust and enhanced release of stress hormones by the adrenal glands, including glucocorticoids, in response to acute stressors could contribute to greater protection against severe COVID-19 and mortality in women.
In this context, it is not surprising that potent glucocorticoids, such as dexamethasone , have been shown to be the most effective therapy currently available in limiting the progression of severe COVID-19 and inflammation.
In an open-label controlled trial of 6,425 hospitalized patients with COVID-19, dexamethasone treatment resulted in lower 28-day mortality in those who received invasive mechanical ventilation or oxygen alone at randomization.69A small, prospective, triple-blind randomized controlled trial (84 patients) demonstrated the superiority of methylprednisolone compared to dexamethasone in terms of clinical status and duration of hospitalization in patients with COVID-19.
In addition to glucocorticoid concentrations , the differential action of cortisol between sexes could contribute to a more favorable response of women to severe COVID-19.
Clinical and public health implications of sex-based differences in COVID-19
Given that men are at higher risk of developing severe COVID-19, the question arises whether older men (≥50 years) with severe comorbidities might require special consideration with respect to prevention, screening, surveillance, and vaccination strategies. In contrast, women appear to be at higher risk for some vaccine-related adverse events, vaccine advancements, and long COVID.
Therefore, a sex-specific approach might be desirable to make optimal recommendations for prevention and treatment strategies in the context of the COVID-19 pandemic. However, we are just beginning to define sex-specific preventive and therapeutic approaches for COVID-19.
Sex-dependent effects of COVID-19 vaccines and reinfections
Data from the past 2 years suggest that sex differences could also have implications for responses to SARS-CoV-2 vaccination and reinfection. Smaller studies propose that COVID-19 reinfections could be associated with greater severity compared to initial infection in both sexes; Additionally, there is evidence that women are more commonly affected by COVID-19 reinfections than men.
The higher rate of reinfection in women is unexpected, since women show a stronger immune response. The reasons for this apparent paradox are unclear, but could be related to the increased antibody responses found in convalescent male plasma donors.
Differences in social behavior during the COVID-19 pandemic would also suggest a greater susceptibility to reinfection in men compared to women. The panel’s evidence suggests that women are more likely to perceive COVID-19 as a very serious health problem and are therefore more likely to agree with and comply with restrictive policies.
A study using mobile phone data from 1.2 million devices in Austria found gender differences in social behavior during different phases of the COVID-19 pandemic; For example, women avoided larger shopping centers during lockdown, and after lockdown, men returned to normal social behavior faster than women.
Sex-related predisposition to long COVID
Another phenomenon with potential sex-related predisposition is long COVID (also called post-COVID syndrome ), which is defined as a complex of persistent nonspecific symptoms, such as chronic fatigue, muscle weakness, sleeping difficulties, anxiety and depression, which are They are observed in individuals after an acute outbreak of COVID-19 and are not explained by other diagnoses.
Post-virus syndromes, including chronic fatigue syndrome, are not uncommon after a variety of viral infections with a long course, for example, caused by cytomegalovirus or Epstein-Barr virus. An increasing number of long COVID cases have been reported in recent months and a female predominance is emerging , similar to chronic fatigue syndrome.
In a cohort study of 5,838 people in Switzerland, women more frequently reported at least one persistent symptom, with reduced resilience being the most common symptom in both men and women. In women, cardiovascular risk factors, pre-existing mental illness, and self-reported household stress increased the risk of long COVID. In addition to female sex, the number of symptoms in the first week, BMI, and increasing age were found to be predictors of long COVID.
In the case of long COVID in particular, as well as any other symptoms reported to the doctor, it is important to note that there may also be sex-related differences in the way symptoms are perceived and reported, which could affect outcomes. results of the studies. For example, women with West Nile virus infection reported significantly more symptoms compared to men despite similar viral load in men and women.
Growing evidence suggests that autoantibodies, whose concentrations also exhibit sex-specific differences, play a crucial role in widespread multiorgan disease in long COVID patients. Pre-existing asthma, which is more common in women than men, has been reported to further increase the risk of developing long COVID.
Further characterization of predictors of long COVID, such as sex and comorbidities, could help identify patients at high risk of developing long COVID and enable early intervention to address their individual needs and improve outcomes.
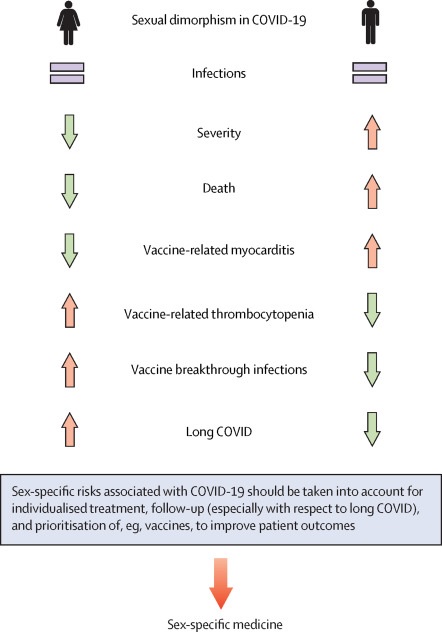
Figure: Sexual dimorphism in severity and mortality of COVID-19. Men experience more severe disease courses and more deaths related to COVID-19, but women appear to be at higher risk for long COVID. Therefore, we argue that sex should be taken into account in the treatment, monitoring and establishment of public health measures of COVID-19.
Conclusion Taken together, there is evidence that sexual dimorphism in COVID-19 has potential implications that should be considered in the treatment of COVID-19 and the follow-up of patients predisposed to the development of long COVID-19, as well as for vaccine prioritization. While COVID-19 infections are more frequently associated with a severe course and higher mortality in men , women appear to be predisposed to long COVID. Although the overall molecular mechanisms do not differ between males and females, differences in the expression patterns of several cell surface proteins responsible for virus attachment and entry, as well as sex-specific differences in stress and immune response, likely contribute to the sexual dimorphism observed in COVID-19. A reanalysis of our own data regarding sexual dimorphism suggests that male patients have higher expression of ACE2 and inflammatory markers in the coronary tree than female patients with similar cardiovascular diseases. This could further highlight a specific predisposition of men to have a higher susceptibility to severe and fatal COVID-19. Lifestyle and behavioral factors , differences in the presence of comorbidities, and sex-specific risk factors also contribute to sexual dimorphism in COVID-19 and should always be taken into account. Although clear mechanisms are not yet known to explain sexual dimorphism in COVID-19, there are many possible clues, some of which deserve further exploration. |