In The Lancet Respiratory Medicine, Kollengode Ramanathan and colleagues provide excellent recommendations for the use of extracorporeal membrane oxygenation (ECMO) for patients with respiratory failure from acute respiratory distress syndrome (ARDS) secondary to coronavirus disease 2019 (COVID-19). ).
The authors describe pragmatic approaches to the challenges of administering ECMO to patients with COVID-19, including training healthcare personnel, troubleshooting equipment and facilities, implementing systems for infection control, and personal protection. , general support to healthcare personnel and mitigating ethical issues.
They also address some of the anticipated challenges with local and regional surges in COVID-19 ARDS cases; Although there has been an increase in hospitals with the capacity to provide ECMO, potential demand could exceed available resources. Additionally, some healthcare systems offer advanced therapies such as ECMO, but lack a coordinated local, regional, or national referral protocol.
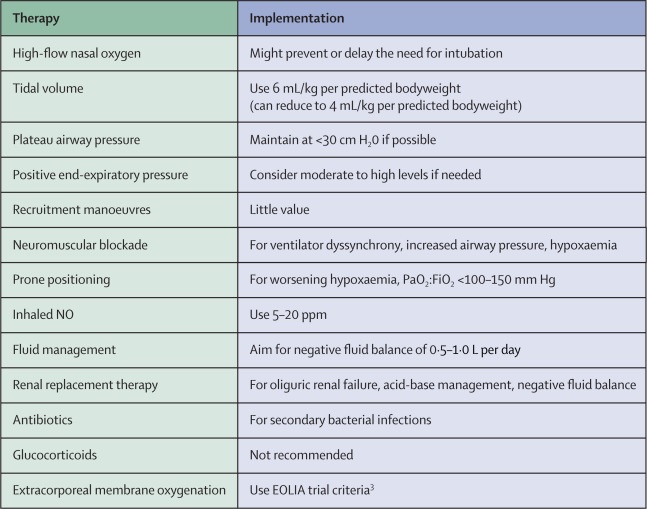
Given the practical limitations to substantially increasing the global availability of ECMO services in the coming months, it is important to emphasize the other evidence-based treatment options that can be provided for patients with severe COVID-19 ARDS (figure).
Before endotracheal intubation, it is important to consider a trial of high-flow nasal oxygen for patients with moderately severe hypoxemia.
This procedure could avoid the need for intubation and mechanical ventilation because it provides high concentrations of humidified oxygen, low levels of positive end-expiratory pressure, and may facilitate the removal of carbon dioxide.
WHO guidelines support the use of high-flow nasal oxygen in some patients, but urge close monitoring to detect clinical deterioration that could lead to the need for emergent intubations because such procedures could increase the risk of infection for patients. health workers.
 Therapeutic options for severe acute respiratory distress syndrome related to coronavirus disease 2019 (original in English)
Therapeutic options for severe acute respiratory distress syndrome related to coronavirus disease 2019 (original in English)
For patients with COVID-19 requiring endotracheal intubation, use of low tidal volume (6 mL/kg per predicted body weight) with a plateau airway pressure of less than 30 cm H2O, and increasing respiratory rate to 35 breaths per minute, as needed, is the mainstay of lung protective ventilation.
If hypoxemia progresses to a PaO2:FiO2 ratio of less than 100–150 mm Hg, several therapeutic options are available. The positive end-expiratory pressure level can be increased by 2–3 cm H2O every 15–30 min to improve oxygen saturation to 88–90%, with the goal of maintaining a plateau airway pressure of less than 30 cm H2O. Lower driving pressures (plateau airway pressure minus positive end-expiratory pressure) can also be used with a goal of 13-15 cm H2O.
If the patient does not respond to adjustment of the positive end-expiratory pressure level, additional strategies may stabilize them. Recruitment maneuvers are probably of little value, but moderate pressures of approximately 30 cm H2O can be applied for 20-30 s in the presence of a physician to monitor hemodynamics.
If there is no improvement in oxygenation or driving pressure, or if the patient develops hypotension or barotrauma, recruitment maneuvers should be discontinued.
If there is considerable dyssynchrony with positive pressure ventilation, accompanied by increased plateau airway pressure and refractory hypoxemia, deep sedation followed by prompt institution of neuromuscular blockade with cisatracurium should be used. Additionally, prone positioning should be instituted, unless there is a specific contraindication, and can be initiated in conjunction with the interventions already described.
For persistent refractory hypoxemia even with prone positioning, neuromuscular blockade, and efforts to optimize positive end-expiratory pressure therapy, there are additional options.
Inhalation of 5–20 ppm NO could improve oxygenation. Insertion of an esophageal balloon to measure transpulmonary pressures to establish an optimal end-expiratory pressure may be considered in patients with moderate to severe obesity, although a 2019 trial in patients with ARDS did not show benefit of this procedure in most patients. patients.
It is important to consider fluid management as a measure to reduce pulmonary edema. In the absence of shock, conservative fluid therapy is recommended to achieve a negative fluid balance of 0.5 to 1.0L per day.
In the presence of shock, fluid balance can be achieved with renal replacement therapy , especially if there is associated acute kidney injury and oliguria.
Antibiotics should be considered as secondary bacterial infections have been reported in patients with COVID-19.
Glucocorticoids should be avoided in view of evidence that they may be harmful in cases of viral pneumonia and influenza ARDS.
Rescue therapy with high doses of vitamin C may also be considered.
Finally, ECMO should be considered using the EOLIA trial inclusion and exclusion criteria.
As treatment of severe COVID-19 ARDS is an ongoing challenge, it is important to learn from patients who have been treated to understand the epidemiology of the disease, biological mechanisms, and the effects of new pharmacological interventions.
There are currently some research groups working to coordinate and disseminate key information, including information on patients who have been treated with ECMO for COVID-19, although a precise estimate of the number of such patients is currently not available. The Extracorporeal Life Support Organization is an international nonprofit consortium that plans to maintain a patient registry to facilitate a better understanding of how ECMO is being used for COVID-19 patients.
MAM reports grants from the National Institutes of Health: National Heart, Lung, and Blood Institute, US Food and Drug Administration, US Department of Defense, Bayer Pharmaceuticals, Genentech -Roche and personal fees from Gen1e Life Sciences, outside of submitted work JMA has served on the electronic medical records committee of the Society of Critical Care Medicine, outside of submitted work. JEG declares that it has no competing interests.