Sabizabulin is a novel orally available microtubule disruptor that targets, binds, and cross-links α- and β-tubulin subunits to inhibit polymerization and induce depolymerization of microtubules in cells. Microtubules are intracellular transport structures critical for the entry, trafficking, replication and egress of coronavirus cells as well as for triggering the innate inflammatory response and cytokine storm responsible for acute respiratory distress syndrome (ARDS), the septic shock and often death.
ARDS is one of the main causes of mortality in Covid-19 infection, since the infiltration of immune cells in both lungs causes lesions and edema in the alveolar-capillary membrane; Increased lung permeability leads to exudates filling the alveoli, with resulting hypoxemia. Preclinical studies demonstrate that sabizabulin has significant antiviral and anti-inflammatory activities by altering microtubule dynamics.
Background
Sabizabulin is a novel oral microtubule disruptor that has dual antiviral and anti-inflammatory activities in preclinical models.
Methods
A phase 3, multicenter, randomized, placebo-controlled clinical trial was conducted with hospitalized patients with moderate to severe Covid-19 who were at high risk of acute respiratory distress syndrome (ARDS) and death.
Patients were randomly assigned (2:1) to 9 mg oral sabizabulin or placebo daily (up to 21 days).
The primary endpoint was all-cause mortality through day 60.
Key secondary endpoints were days in the intensive care unit (ICU), days on mechanical ventilation, and days in the hospital.
Results
A total of 204 patients were randomly assigned to treatment: 134 to sabizabulin and 70 to placebo. Baseline characteristics were similar.
The superiority of sabizabulin was demonstrated by a planned interim analysis for the first 150 randomized patients. Treatment with sabizabulin resulted in an absolute reduction of 24.9 percentage points and a relative reduction of 55.2% in deaths compared with placebo (odds ratio, 3.23; 95% CI, confidence interval, 1 .45 to 7.22; P = 0.0042).
The mortality rate was 20.2% (19 of 94) for sabizabulin versus 45.1% (23 of 51) for placebo.
For key secondary endpoints, sabizabulin treatment resulted in a 43% relative reduction in ICU days (P = 0.0013), a 49% relative reduction in mechanically ventilated days (P = 0.0013), 0013) and a 26% relative reduction in hospital days (P=0.0277) versus placebo.
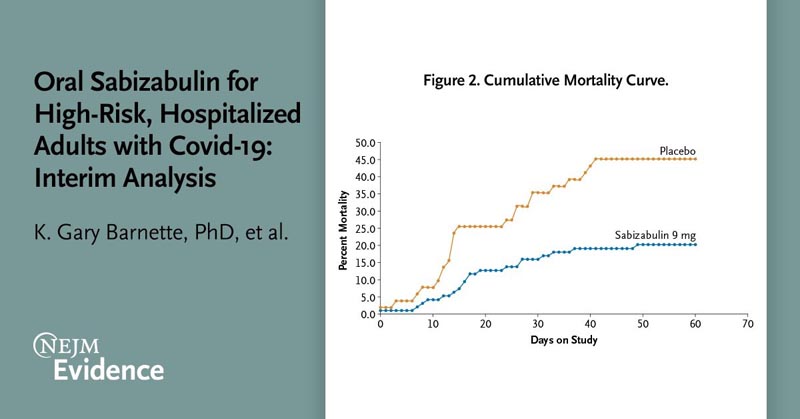
Conclusions Treatment with sabizabulin resulted in a 24.9% absolute reduction in deaths compared to placebo in hospitalized patients with moderate to severe Covid-19 at high risk of ARDS and death, with a lower incidence of serious adverse events and adverse events compared to placebo. |
Discussion
Vaccines remain the mainstay for the prevention of serious infections and deaths from Covid-19 . Most patients will recover from acute Covid-19 illness. The new antiviral agents molnupiravir and nirmatrelvir reduce the incidence of Covid-19-related hospitalizations or deaths when taken in a non-hospital setting within 3 to 5 days of the onset of Covid-19 symptoms. For patients who progress to moderate to severe COVID-19 illness requiring hospitalization, the risk of death remains high. In this context, however, the antiviral molnupiravir did not demonstrate clinical benefit.
Sabizabulin is a new microtubule disruptor that has dual antiviral and anti-inflammatory activities. Sabizabulin appears to be a member of a new class of drugs that targets, binds, and cross-links the α- and β-tubulin subunits of microtubules to inhibit polymerization and induce depolymerization of microtubules, thereby altering microtubule dynamics. Sabizabulin has a different chemical structure and unique physicochemical properties compared to the more familiar drug, colchicine . Unlike colchicine, sabizabulin is not a substrate for P-glycoprotein or CYP3A4, which may result in higher and more consistent intracellular and blood concentrations of sabizabulin than colchicine. Therefore, sabizabulin has a different chemical structure and properties. unique physicochemical characteristics compared to colchicine.
Daily oral dosing of 9 mg sabizabulin for up to 21 days demonstrated significant efficacy in this interim analysis of a global, randomized, double-blind, placebo-controlled Phase 3 clinical trial in hospitalized adult patients with moderate to severe Covid-19 who were at high risk of ARDS and death. Sabizabulin demonstrated a statistically significant 24.9% absolute reduction and 55.2% relative reduction in all-cause mortality at day 60, the study’s primary efficacy endpoint.
Cumulative mortality analysis showed that the reduction in deaths with sabizabulin began in the first week of treatment and the relative reduction in deaths reached 51.8% at day 29. Significantly fewer serious adverse events and adverse events were reported for sabizabulin in comparison with placebo. There were also fewer treatment discontinuations due to adverse events in the sabizabulin group compared to placebo.
In this study, sabizabulin was evaluated in hospitalized patients with moderate to severe Covid-19 disease. Inclusion criteria were selected by design to include patients with the highest risk of ARDS and death from Covid-19. Enrolled hospitalized patients had to demonstrate moderate to severe Covid-19 disease (at least oxygen supplementation with SpO2 of 94% or less while breathing room air). Patients with a WHO score of 4 (receiving supplemental oxygen) had to have a comorbidity that put them at high risk of death; and no limitation was placed on the duration of Covid-19 symptoms prior to enrollment.
Consequently, the mortality rate in the placebo group for this study was 35.3% at day 29; by day 60, the mortality rate in this group increased further to 45.1%. It is evident that the mortality rate of patients hospitalized with moderate to severe Covid-19 remains high even with available therapies, such as the antiviral agent remdesivir, immunomodulators or anti-inflammatory agents. By targeting microtubule trafficking, sabizabulin has dual anti-inflammatory and antiviral activity. These data demonstrate that sabizabulin treatment significantly reduced mortality with an acceptable safety and side effect profile in hospitalized patients with moderate to severe Covid-19 at high risk of ARDS.