Researchers at the University of Illinois at Chicago are the first to identify the presence of a specific type of antibody, called anti-citrullinated protein autoantibodies , or ACPA, in human tear fluid. They are also the first to demonstrate that patients with dry eye disease experienced reduced signs and symptoms of the condition in response to a new eye drop treatment, made of pooled human antibodies, that targets ACPAs.
Findings from their early-stage clinical trial are reported in the journal The Ocular Surface .
Dry eye disease is caused by abnormalities in tear fluid and results in dry areas over the cornea, the transparent outer layer of the eye, which can cause disabling eye pain and sensitivity to light in severe cases.
"The burden of autoimmune dry eye is much greater than having an occasional feeling of dryness," said Dr. Sandeep Jain, senior author of the study and UIC professor of ophthalmology and visual sciences in the School of Medicine. "It can severely compromise quality of life to the point of disability and can compromise a person’s vision."
In previous research, Jain and his colleagues found that DNA strands are extruded from neutrophils, a type of white blood cell, to form networks on the surface of eyes affected by severe dry eye disease and cause inflammation.
In the new study, researchers identified ACPAs as another cause of ocular inflammation that also contributes to the development of these networks, which Jain calls "a vicious cycle of inflammation."
The new eye drops treat dry eye disease by removing the immune system from this cycle, at least partially.
The drops are formulated using pooled antibodies, which are made of immunoglobulins processed from the donated blood of thousands of individuals, all with different types of antibodies, which counteract the negative effects of ACPAs.
The phase I/II drug trial compared antibody-based eye drops to eye drops without the antibodies.
"Currently, there are only two drugs approved to treat dry eye, and they don’t work for everyone, especially those with severe disease, so having a new drug that can treat the disease through a different mechanism, in this case, autoimmunity, It’s very important," Jain said.
Twenty-seven participants with severe dry eye disease participated in the trial. The participants were randomly assigned into two groups. One group was given eye drops made from pooled antibodies and instructed to administer one drop to each eye twice a day for eight weeks. The control group received the same instructions with eye drops made without antibodies.
The researchers assessed patients’ symptoms through questionnaires and measured the extent of corneal damage and the amount of pro-inflammatory biomarkers on the surface of the eye before and during the duration of the study.
They found that people using antibody-based eye drops had a statistically significant and clinically significant reduction in corneal damage at eight weeks compared to the control group.
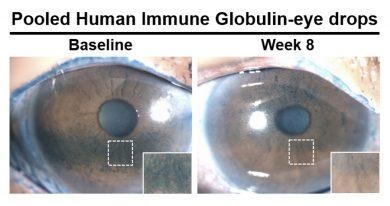
Symptom-related questionnaire scores also reflected significant improvement among patients using the new antibody-based eye drops compared to non-antibody eye drops. In the test group, the number of pro-inflammatory biomarkers, or dry areas, was also reduced on the surface of the eye.
"Trial participants who used the drops with clustered antibodies reported less eye discomfort and their corneas were healthier," Jain said. "Data from this early clinical trial suggest that eye drops containing clustered antibodies may be safe and effective in treating dry eye disease, and we hope to conduct larger randomized trials to definitively demonstrate their effectiveness."
Co-authors of the study are Jieun Kwon, Bayasgalan Surenkhuu, Ilangovan Raju, Nour Atassi, Jessica Mun, Yi-Fan Chen, Monazzah Akbar Sarwar, Mark Rosenblatt, Anubhav Pradeep, Seungwon An, Nikhil Dhal and Christine Mun of UIC.
This research was supported by the National Eye Institute (R01EY024966, P30EY001792) and research awards from Research to Prevent Blindness and UIC. Jain is the inventor of a patent assigned to UIC covering the use of immunoglobulin eye drops in dry eye disease.