
The organization’s latest clinical practice guideline Kidney Disease: Improving Global Outcomes (KDIGO) offers advice for treating patients with diabetes and chronic kidney disease (CKD). A synopsis published in the Annals of Internal Medicine focuses on key recommendations relevant to the following topics: comprehensive care, glycemic control and goals, lifestyle interventions, antihyperglycemic therapies, and educational and integrated care approaches to management.
The guideline is designed to apply to a broad population of patients with diabetes and CKD, taking into account implications for policy and payment. Both type 1 and type 2 diabetes are addressed, and differences in management approach are highlighted where appropriate.
The KDIGO guideline update is based on literature searches last conducted in December 2021, limiting searches to randomized controlled trials only, and updated these searches in February 2022 at the time of public review. The evidence synthesis and meta-analysis methods performed for the 2020 KDIGO Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease were followed for the 2022 guideline update.
The KDIGO working group authors summarized the guideline update to facilitate reference in clinical practice. The update recommends:
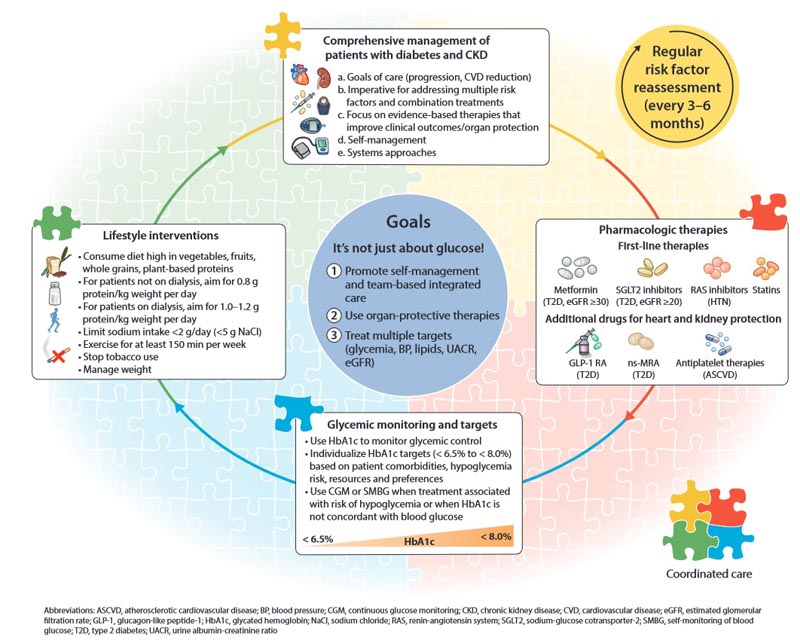
A stepped approach to care, starting with a foundation of lifestyle interventions and first-line pharmacotherapy demonstrated to improve clinical outcomes.
The serial introduction of drugs that improve intrarenal hemodynamics (such as RAS inhibitors, SGLT2 inhibitors, MRAs, diuretics and other antihypertensive drugs).
That healthcare providers should focus on preserving kidney function and maintaining well-being rather than replacing kidney function.
That policymakers and institutional decision makers implement integrated team-based care focused on risk assessment and patient empowerment to provide comprehensive care for patients with diabetes and CKD.
Points for clinical practice
1.1.1: Patients with diabetes and chronic kidney disease (CKD) should be treated with a comprehensive strategy to reduce the risks of progression of kidney disease and cardiovascular disease.
SGLT2 inhibitors
Recommendation 1.3.1 : We recommend treating patients with type 2 diabetes (T2D), CKD and an eGFR ‡20 ml/min per 1.73 m2 with an SGLT2i (1A).
Practice Point 1.3.1 : The recommendation for SGLT2i is for renal and cardiovascular protection and SGLT2i has been shown to be safe and beneficial in patients with CKD, including those without T2D. Therefore, if patients are already being treated with other glucose-lowering agents, an SGLT2i can be added to the current treatment regimen.
Practice Point 1.3.2 : The choice of an SGLT2i should prioritize agents with documented renal or cardiovascular benefits and take eGFR into account.
Practice Point 1.3.3 : It is reasonable to discontinue SGLT2i during times of prolonged fasting, surgery, or critical medical illness (when patients may be at increased risk for ketosis).
Practice Point 1.3.4 : If a patient is at risk for hypovolemia , consider decreasing doses of thiazides or loop diuretics before starting treatment with SGLT2i, advise patients about symptoms of volume depletion and low blood pressure, and track volume status after starting the drug.
Practice Point 1.3.5 : A reversible decrease in eGFR may occur with initiation of SGLT2i treatment and is generally not an indication to discontinue therapy.
Practice Point 1.3.6 : Once an SGLT2i is started, it is reasonable to continue with an SGLT2i even if the eGFR falls below 20 ml/min per 1.73 m2 , unless it is not tolerated or a renal replacement therapy.
Practice Point 1.3.7 : SGLT2i has not been adequately studied in kidney transplant recipients, who may benefit from SGLT2i treatment but are immunocompromised and have a potentially increased risk of infections; therefore, the recommendation to use SGLT2i does not apply to kidney transplant recipients (see Recommendation 1.3.1).
Mineralocorticoid receptor antagonists (MRA)
Recommendation 1.4.1 : We suggest a non-steroidal mineralocorticoid receptor antagonist with proven renal or cardiovascular benefit for patients with T2D, an eGFR ‡25 ml/min per 1.73 m2, normal serum potassium concentration and albuminuria (‡30 mg /g [‡3 mg/mmol]) despite the maximum tolerated dose of RAS inhibitor (RASi) (2A).
Practice Point 1.4.1 : Non-steroidal MRAs are most appropriate for patients with T2D who are at high risk of CKD progression and cardiovascular events, as evidenced by persistent albuminuria despite other standard of care therapies.
Practice Point 1.4.2 . A non-steroidal ARM can be added to a RASi and SGLT2i for the treatment of T2D and CKD. Practice Point 1.4.3. To mitigate the risk of hyperkalemia, select patients with a consistently normal serum potassium concentration and monitor serum potassium regularly after initiation of nonsteroidal MRA. Practice
Practice Point 1.4.4 . The choice of a nonsteroidal MRA should prioritize agents with documented renal or cardiovascular benefits.
Practice Point 1.4.5 . A steroid-based MRA should be used for the treatment of heart failure, hyperaldosteronism, or refractory hypertension, but may cause hyperkalemia or a reversible decrease in glomerular filtration, particularly in patients with a low GFR. GLP-1 receptor agonists
Recommendation 4.2.1 : In patients with T2D and CKD who have not achieved individualized glycemic goals despite the use of metformin and SGLT2i treatment, or who are unable to use these medications, we recommend a long-acting GLP-1 RA (1B ).
Practice Point 4.2.1 : The choice of GLP-1 RA should prioritize agents with documented cardiovascular benefits.
Practice Point 4.2.2 : To minimize gastrointestinal side effects, start with a low dose of GLP-1 RA and increase slowly.
Practice Point 4.2.3 : GLP-1 RA should not be used in combination with dipeptidyl peptidase-4 (DPP-4) inhibitors.
Practice Point 4.2.4 : The risk of hypoglycemia is generally low with GLP-1 RA when used alone, but the risk increases when GLP-1 RA is used concomitantly with other medications such as sulfonylureas or insulin. Sulfonylurea and/or insulin doses may need to be reduced.
Practice Point 4.2.5 . GLP-1 RA can be preferentially used in patients with obesity, T2D and CKD to promote intentional weight loss.
The full updated clinical guideline can be accessed at https://kdigo.org/guidelines/diabetes-ckd .
















