The Third Blood Pressure Lowering Intensive Care Bundle in Acute Cerebral Hemorrhage Trial (INTERACT3): An International Stepped Cluster Randomized Controlled Trial
Background
Early control of elevated blood pressure is the most promising treatment for acute intracerebral hemorrhage. Our objective was to establish whether implementation of a goal-directed care package incorporating protocols for early intensive blood pressure reduction and management algorithms for hyperglycemia, pyrexia and abnormal anticoagulation , implemented in a hospital setting, could improve outcomes for patients with acute spontaneous intracerebral hemorrhage.
Methods
We conducted a pragmatic, international, multicenter, endpoint-blind, stepped-wedge cluster randomized controlled trial in hospitals in nine low- and middle-income countries (Brazil, China, India, Mexico, Nigeria, Pakistan, Peru, Sri Lanka, and Viet Nam) and a high-income country (Chile). Hospitals were eligible if they had no relevant or inconsistent disease-specific protocols, and were willing to implement the care package to consecutive patients (age ≥ 18 years) with imaging-confirmed spontaneous intracerebral hemorrhage presenting within 6 h of onset of symptoms. had a local champion and could provide the required study data.
Hospitals were centrally randomized using permuted blocks to three implementation sequences, stratified by country and the projected number of patients to be enrolled during the 12-month study period. These sequences had four periods that dictated the order in which hospitals were to shift from the control usual care procedure to implementing the care package procedure intervention to different patient groups in a stepwise manner. To avoid contamination, details of the intervention, sequence, and allocation periods were hidden from sites until they had completed the usual attention control periods.
The care package protocol included:
|
Analyzes were performed according to a modified intention-to-treat population with available outcome data (i.e., excluding sites that were withdrawn during the study). The primary outcome was functional recovery, measured with the modified Rankin Scale (mRS; range 0 [no symptoms] to 6 [death]) at 6 months by masked research personnel, analyzed using proportional ordinal logistic regression to assess the distribution of scores on the mRS, with adjustments for cluster (hospital site), cluster group assignment by period and time (6-month periods since December 12, 2017). This trial is registered in patients where these variables were abnormal .
Results
Between May 27, 2017 and July 8, 2021, 206 hospitals were assessed for eligibility, of which 144 hospitals in ten countries agreed to join and were randomized into the trial, but 22 hospitals withdrew before starting. to enroll patients and another hospital was withdrawn and its data on enrolled patients deleted because regulatory approval was not obtained.
Between December 12, 2017 and December 31, 2021, 10,857 patients were evaluated, but 3,821 were excluded. Overall, the modified intention-to-treat population included 7,036 patients enrolled at 121 hospitals, with 3,221 assigned to the bundle group care and 3815 to the usual care group, with primary outcome data available on 2892 patients in the care package group and 3363 patients in the usual care group.
The likelihood of poor functional outcome was lower in the care bundle group (common odds ratio 0.86, 95% CI 0.76–0.97, p=0.015). The favorable change in mRS scores in the care bundle group was generally consistent across a variety of sensitivity analyzes that included additional adjustment for country and patient variables (0.84, 0.73–0.97, p = 0.017), and with different approaches to using multiple imputations for missing data.
Patients in the bundle of care group had fewer serious adverse events than those in the usual care group (16.0% vs. 20.1%; p=0.0098).
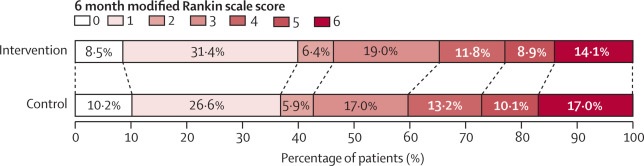
Functional outcome at 90 days in the care package and usual care groups, according to mRS scores . Raw distribution of mRS scores at 90 days. Scores on the mRS range from 0 to 6, where 0 indicates no symptoms, 1 indicates symptoms without clinically significant disability, 2 indicates mild disability, 3 indicates moderate disability, 4 indicates moderately severe disability, 5 indicates severe disability, and 6 indicates death. . There was a significant difference between the care bundle group and the usual care group in the overall distribution of scores (common odds ratio, indicating a lower probability of worse overall function outcome on the mRS, 0.86 [ 95% CI 0.76–0 ·97], p=0.015). mRS=Modified Rankin Scale.
Interpretation
Implementation of a care bundle protocol for intensive blood pressure lowering and other management algorithms for physiologic control within several hours of symptom onset resulted in improved functional outcome for patients with acute intracerebral hemorrhage. Hospitals should incorporate this approach into clinical practice as part of the active management of this serious condition.
Comments
Combining readily available treatments improved outcomes, with particular benefit for low- and middle-income countries, where most cases occur.
The George Institute for Global Health announced data from the Phase III INTERACT3 study showing that a new combination of treatments for stroke due to intracerebral hemorrhage (ICH) significantly improves the chances of survival without major disability. The results were presented today at the European Stroke Organization Conference in Munich, Germany, and were simultaneously published in The Lancet .
The INTERACT3 study is the first randomized controlled trial to show a clearly positive outcome for the treatment of ICH. Timely administration of the new treatment protocol, known as the Care Bundle , focused on rapid control of high blood pressure, led to better recovery, lower death rates, and better overall quality of life in patients with this serious condition.
Professor Craig Anderson, Director of Global Brain Health at The George Institute and lead author of the research, said: "Despite the high rates of intracerebral haemorrhage (ICH) and its severity, there are few proven options to treat it, but control early treatment of high blood pressure is critical. Time is critical when treating this type of stroke, so we tested a combination of interventions to quickly stabilize these patients’ condition to improve their outcomes. We estimate that, if this protocol were universally adopted , it could save tens of thousands of lives each year around the world .”
Commonly known as hemorrhagic stroke or brain hemorrhage, intracerebral hemorrhage (ICH) is the second most common type of stroke and also the deadliest, with 40% to 50% of patients dying within 30 days. It occurs when blood leaks from a blood vessel into brain tissue and accounts for more than a quarter of all stroke cases, affecting approximately 3.4 million people a year.
In the INTERACT3 study, more than 7,000 patients were enrolled in 144 hospitals in 10 countries: nine middle-income countries and one high-income country. The research team evaluated the effectiveness of the new Care Bundle , which included early and intensive reduction of systolic blood pressure, tight glucose control, treatment of fever, and rapid reversal of abnormal anticoagulation .
They found that using this new treatment protocol compared to usual care reduced the likelihood of poor functional outcome, including death, after six months. It was estimated that this would prevent one additional death for every 35 patients treated.
Central to this was a rapid reduction in systolic blood pressure, with target levels being reached, on average, in 2.3 hours [range 0.8 to 8.0 hours], compared to 4.0 hours [range 1.9 to 16.0 hours] in the control group. The intervention protocol resulted in a statistically significant reduction in mortality , the number of serious adverse events and hospitalization time, in addition to demonstrating an improvement in health-related quality of life.
The burden of intracerebral hemorrhage (ICH) is highest in low- and middle-income countries (LMICs). In 2019, 30% of all stroke cases in LMICs were ICH, almost double the proportion seen in high-income countries (16%). This is partly due to high rates of high blood pressure and limited resources for primary prevention strategies, including the identification and management of stroke risk factors by healthcare services.
Dr Lili Song, joint lead author and head of the Stroke Program at the George Institute of China, said: “The lack of proven treatments for intracerebral hemorrhage (ICH) has led to a pessimistic view that not much can be done. for these patients. However, with INTERACT3, we demonstrate at scale how readily available treatments can be used to improve outcomes in resource-limited settings. “We hope this evidence informs clinical practice guidelines around the world and helps save many lives.”
Added value of this study
The third trial of the Intensive Care Bundle with Blood Pressure Lowering in Acute Cerebral Hemorrhage is the only randomized controlled trial that includes a care bundle that included intensive blood pressure-lowering treatment in acute intracerebral hemorrhage.
The primary outcome was that implementation of the care bundle at participating hospitals resulted in patients having a better functional outcome (measured on the modified Rankin Scale) at 6 months post-treatment compared to usual care. This outcome included favorable effects on survival and health-related quality of life. The results could not be explained by temporal trends in patient characteristics or management.
Implications of all available evidence
A simple, time-sensitive, goal-directed care bundle protocol , with a core strategy of early intensive blood pressure control to a systolic blood pressure goal of less than 140 mm Hg , was safe and effective in improving outcome functional of acute intracerebral disease. hemorrhage. These results provide a clear implication for rapid control of blood pressure and other physiological variables to be incorporated into clinical practice as part of the active management of this serious disease.
This trial is registered with Clinicaltrials.gov (NCT03209258) and China Clinical Trials Registry (ChiCTR-IOC-17011787) and is complete.
















