Weekly Icodec versus daily Glargine U100 in type 2 diabetes without prior insulin
Background
Insulin Icodec is an investigational basal insulin analog administered once a week for diabetes management.
Methods
We conducted a 78-week, open-label, randomized Phase 3a trial (including a 52-week main phase and a 26-week extension phase, plus a 5-week follow-up period) involving adults with type 2 diabetics (glycated hemoglobin level, 7 to 11%) who had not previously received insulin.
Participants were randomly assigned in a 1:1 ratio to receive insulin icodec once a week or insulin glargine U100 once a day.
The primary endpoint was the change in glycated hemoglobin level from baseline to week 52; The confirmatory secondary endpoint was the percentage of time spent in the glycemic range of 70 to 180 mg per deciliter (3.9 to 10.0 mmol per liter) at weeks 48 to 52. Episodes of hypoglycemia (from the beginning until weeks 52 and 83).
Results
Each group included 492 participants . Baseline characteristics were similar in the two groups. The mean reduction in glycated hemoglobin level at 52 weeks was greater with icodec than with glargine U100 (from 8.50% to 6.93% with icodec [mean change, −1.55 percentage points] and 8.44 % to 7.12% with glargine U100 [mean change, −1.35 percentage points]); The estimated difference between groups (−0.19 percentage points; 95% confidence interval [CI], −0.36 to −0.03) confirmed noninferiority (P<0.001) and superiority (P=0.03). 02) from icodec.
The percentage of time spent in the glycemic range of 70 to 180 mg per deciliter was significantly greater with icodec than with glargine U100 (71.9% vs. 66.9%; estimated difference between groups, 4.27 percentage points [CI 95%, 1.92 to 6.62]; P<0.001), which confirmed the superiority.
The rates of combined severe or clinically significant hypoglycemia were 0.30 events per person-year of exposure with icodec and 0.16 events per person-year of exposure with glargine U100 at week 52 (estimated rate ratio, 1.64; 95% CI, 0.98 to 2.75) and 0.30 and 0.16 events per person-year of exposure, respectively, at week 83 (estimated rate ratio, 1.63; 95% CI, 1.02 to 2.61).
No new safety signals were identified and the incidence of adverse events was similar in the two groups.
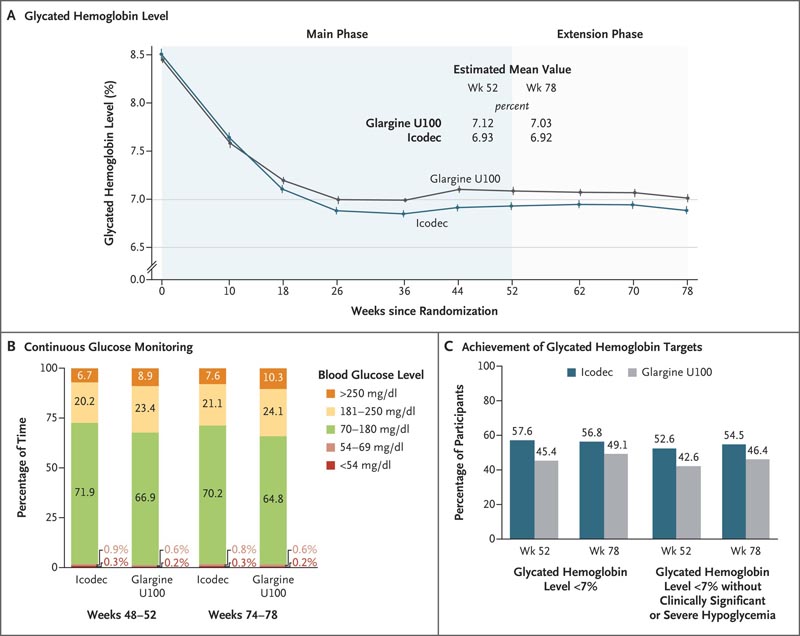
Figure. Key Final Points . Panel A shows mean glycated hemoglobin levels from baseline to week 78 among participants receiving insulin icodec once weekly or insulin glargine U100 once daily. The estimated mean values at weeks 52 and 78 were derived on the basis of multiple imputation. To convert glycosylated hemoglobin values to millimoles per mole, multiply by 10.93 and then subtract 23.50. Error bars indicate standard errors. Panel B shows the percentage of time spent at various glucose levels at weeks 48 to 52 and weeks 74 to 78 as measured by continuous glucose monitoring. The target glycemic range was 70 to 180 mg per deciliter (3.9 to 10.0 mmol per liter). To convert glucose values to millimoles per liter, multiply by 0.05551. Panel C shows the estimated percentage of participants who achieved a glycated hemoglobin level less than 7% and the percentage who achieved a glycated hemoglobin level less than 7% without severe or clinically significant hypoglycemia in the previous 12 weeks. Clinically significant hypoglycemia (level 2) is defined as a blood glucose level of less than 54 mg per deciliter (<3.0 mmol per liter), confirmed with a blood glucose meter. Severe hypoglycemia (level 3) is defined as hypoglycemia with severe cognitive impairment requiring outside assistance for recovery.
Conclusions Glycemic control was significantly better with insulin icodec once weekly than with insulin glargine U100 once daily. |
Reference : NEJM DOI: 10.1056/NEJMoa2303208
(Funded by Novo Nordisk; HEREafter 1 issue of ClinicalTrials.gov, NCT04460885. opens in new tab.)