Aim
To develop a risk assessment tool to identify patients with type 2 diabetes (T2D) at highest risk of kidney disease progression and who could benefit most from sodium-glucose cotransporter 2 (SGLT2) inhibition.
Methodology
A total of 41,204 T2D patients from four thrombolysis in myocardial infarction (TIMI) clinical trials were divided into derivation (70%) and validation cohorts (30%).
Candidate predictors of kidney disease progression (composite of sustained ≥40% decline in estimated glomerular filtration rate [eGFR], end-stage renal disease, or renal death) were selected with multivariable Cox regression.
The efficacy of dapagliflozin was evaluated by risk categories (low: <0.5%; intermediate: 0.5 to <2%; high: ≥2%) in Dapagliflozin Effect on Cardiovascular Events (DECLARE)-TIMI 58.
Results
There were 695 events during a median follow-up of 2.4 years .
The final model comprised eight independent predictors of kidney disease progression: atherosclerotic cardiovascular disease, heart failure, systolic blood pressure, duration of type 2 diabetes, glycated hemoglobin, eGFR, urine albumin-to-creatinine ratio, and hemoglobin.
The c-indexes were 0.798 (95% CI, 0.774–0.821) and 0.798 (95% CI, 0.765–0.831) in the derivation and validation cohort, respectively. The slope of the calibration plot (deciles of predicted risk versus observed risk) was 0.98 (95% CI, 0.93–1.04) in the validation cohort.
While relative risk reductions with dapagliflozin did not differ between risk categories, there was a greater absolute risk reduction in patients with a higher baseline risk, at 3.
5% absolute risk reduction in kidney disease progression at 4 years in the highest risk group (≥1%/year). The results were similar with the 2022 Chronic Kidney Disease Prognosis Consortium risk prediction model.
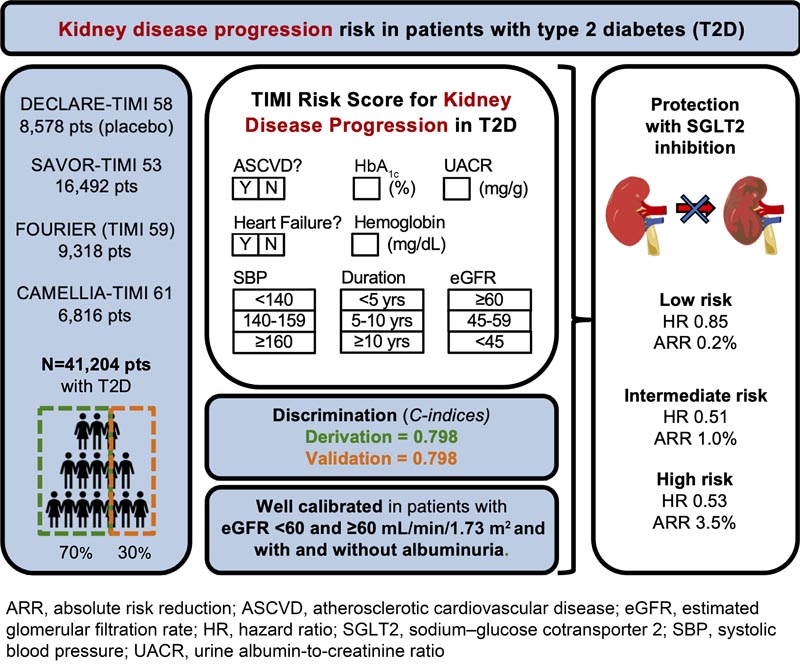
Conclusions Risk models for kidney disease progression in patients with type 2 diabetes can be applied to stratify risk and identify those who experience a greater magnitude of benefit with SGLT2 inhibition. |