
Comparative Effectiveness of Aspirin Dosing in Cardiovascular Diseases
Background
The appropriate dose of aspirin to reduce the risk of death, myocardial infarction, and stroke and to minimize major bleeding in patients with established atherosclerotic cardiovascular disease is a matter of controversy.
Methods
Using a pragmatic open-label design, we randomly assigned patients with established atherosclerotic cardiovascular disease to a strategy of 81 mg or 325 mg of aspirin per day.
The primary effectiveness outcome was a composite of death from any cause, hospitalization for myocardial infarction, or hospitalization for stroke, assessed in a time-to-event analysis.
The primary safety outcome was hospitalization for major bleeding, also assessed in a time-to-event analysis.
Results
A total of 15,076 patients were followed for a median of 26.2 months (interquartile range [IQR], 19.0 to 34.9). Before randomization, 13,537 (96.0% of those with information available on prior aspirin use) were already taking aspirin, and 85.3% of these patients were previously taking 81 mg of aspirin daily.
Death, hospitalization for myocardial infarction, or hospitalization for stroke occurred in 590 patients (estimated percentage, 7.28%) in the 81 mg group and 569 patients (estimated percentage, 7.51%) in the 325 mg group (hazard ratio, 1.02; 95% confidence interval [CI], 0.91 to 1.14).
Hospitalization for major bleeding occurred in 53 patients (estimated percentage, 0.63%) in the 81 mg group and 44 patients (estimated percentage, 0.60%) in the 325 mg group (hazard ratio, 1.18). ; 95% CI, 0.79 to 1.77).
Patients assigned to 325 mg had a higher incidence of dose switching than those assigned to 81 mg (41.6% vs. 7.1%) and fewer days of median exposure to the assigned dose (434 days [IQR, 139 to 737] vs. 650 days [IQR, 415 to 922]).
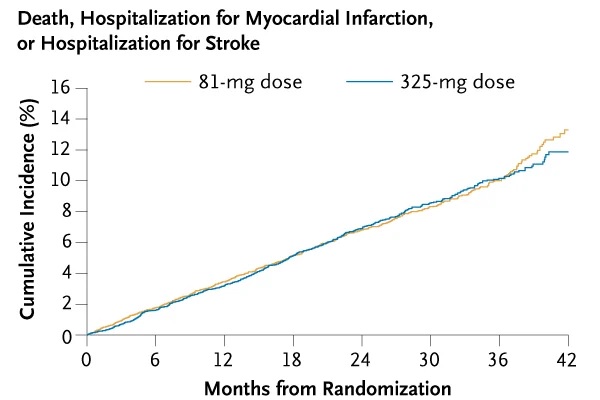
Conclusions In this pragmatic trial involving patients with established cardiovascular disease, there was a substantial dose change to 81 mg of aspirin daily and no significant differences in cardiovascular events or major bleeding between patients assigned to 81 mg and those assigned to 325 mg of aspirin per day. |
(Funded by the Patient-Centered Outcomes Research Institute; ADAPTABLE ClinicalTrials.gov number, NCT02697916. Opens in a new tab.)
















