| Pharmacodermies By Ana Clara Torre (Argentina). |
Any harmful or unwanted effect that appears after the use of a drug at a prophylactic, diagnostic or treatment dose. The WHO estimates that it accounts for around 15% of all adverse drug reactions.
They can be classified as simple or uncomplicated (90-95% of cases), in which mild skin involvement is observed, without systemic involvement, and complex or complicated (2-5% of cases), which present involvement. severe skin and/or systemic involvement. In this last group, laboratory alterations, Nikolsky sign, generalized pustules, target lesions, purpuric lesions or erythroderma are usually seen.
Based on the severity of the condition, they can be addressed in the following way:
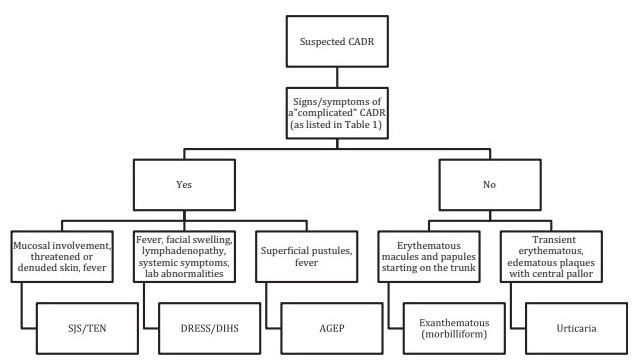
CADR: cutaneous adverse drug reaction
AGEP: acute generalized pustular rash
SJS/TEN: Steven-Johnson syndrome/toxic epidermal necrolysis
DRESS/DIHS: adverse drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome.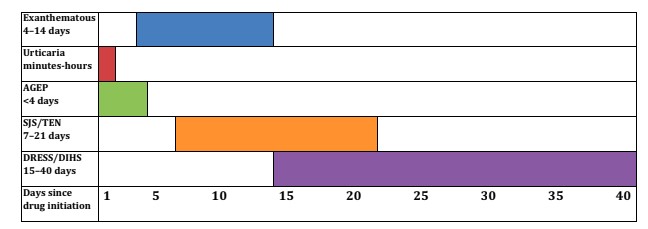
It should be noted that not all symptoms present the same latency time after the introduction of the medication, forcing us to question even those consumed up to 40 days prior to the appearance of the skin condition.
Swanson L, Colven RM. Approach to the Patient with a Suspected Cutaneous Adverse Drug Reaction. Med Clin North Am. 2015 Nov;99(6):1337-48
Regarding the study of these clinical conditions, a laboratory study is essential, both to evaluate target organ damage and to rule out other differential diagnoses.
| Infantile mastocytosis By Prof. Dr. Antonio Torrelo (Spain). |
Set of malignant diseases in which a pathological accumulation of mast cells is observed in different tissues. They are due to a clonal mutation of the kit gene. It can begin at different ages and present different clinical phenotypes, which makes its classification more complex.
The WHO recognizes only 3 subtypes in pediatric patients based on the number of lesions, leaving out certain parameters such as genetics, morphology, histology or the behavior of the disease. This classification generates a lot of controversy, using ambiguous terms, often not being able to classify the patients we evaluate daily.
Torrelo, given his experience on this topic, proposes 2 types of childhood mastocytosis , type 1 and 2. The first, or classic, is the type observed in adults. Type 2 or well differentiated, frequently observed in children.
Type 1 : small, melanic macular or papular skin lesions; variable number of injuries. Dermoscopy shows telengiectasias. Darier + sign but difficult to provoke. Blisters may occasionally be seen. Histopathology shows perivascular spindle mast cells in moderate numbers. It is rarely accompanied by systemic symptoms. Mast cells with mutation of the kit gene at codon 815 or 816. The mast cells of the bone marrow are spindle-shaped and positively mark CD2 and CD25 in flow cytometry. It does not respond to imatinib but does respond to midostaurin.
It is the variety most observed in childhood.
Type 2 : macules, plaques, papules, large nodules and there may be a large number of lesions. Blisters can often be observed. With evolution, anetoderma and erythrodermic forms can be observed. Darier sign + prominent. Histopathology shows round and granulated mast cells, with polylobulated nuclei. Systemic symptoms are common. Bone marrow mast cells mark negatively on flow cytometry for CD2 and CD25. The mutations in the kit are not observed in codon 815 or 816, it responds very well to the tyrosine kinase inhibitor imatinib. This variety corresponds to 5-10% of the cases of mastocytosis observed in adult life, since it has a tendency to disappear.
| Atopic discoid and lichenoid dermatitis By Prof. Dr. José Ollague (Ecuador). |
Ollague proposes (based on a series of patients evaluated in his office) to consider a subvariety of atopic dermatitis (AD) in patients who present discoid and lichenoid plaques , symmetrically located in areas of limb extension, respecting the flexures , accompanied by hyperkeratosis. palmo-plantar. In addition, Ig E is very high in serum, clinically translating as intense accompanying itching . The histopathology of these patients highlights the presence of psoriasiform changes in the epidermis and eosinophils in the dermis .
To differentiate discoid and lichenoid AD from Sulzberger-Garbe syndrome, this subvariety is proposed as an independent entity.
| JAK inhibitors in atopic dermatitis By Dr. Paula Luna (Argentina). |
AD presents a very complex physiopathogenesis, the result of an interaction of multiple actors. It certainly has a genetic basis, a pool of genes, related to the skin barrier, pruritus and immunity. On the other hand, it is important to highlight the role of the microbiome both in the genesis and in the perpetuity of AD.
Today it is known that the origin of AD does not reside solely in the Th2 pathway but also in the Th1, with multiple cytokines involved in addition to the widely known 4 and 13 (inhibited by dupilumab); IL 31 responsible for pruritus, among others.
In clinical practice we observe different AD phenotypes, with differences observed depending on race, evolutionary time and age group, among other factors in question. This could correspond to the inflammatory pathways involved in each particular case. The treatment of this pathology is evolving into a “tailored suit” paradigm, matching the treatment for each patient based on the path(s) involved. The great clinical and physiopathogenic heterogeneity means that there are multiple therapeutic options, varying in effectiveness, from cellular inhibitors to highly specific inhibitors such as biological ones.
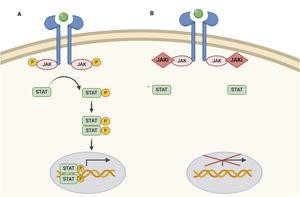
JAK inhibitors are moderately specific, inhibiting several cytokines along the JAK-STAT pathway. They prevent STAT phosphorylation, activation and, therefore, its action at the level of the cell nucleus by modulating genetic transcription.
Dermosifiliographic Minutes January 21, 2021
JAK has 4 subvarieties, being functional in the form of dimers, acting as a receptor for different cytokines and thus activating physiological pathways.
There are 3 JAK inhibitors for systemic use: baricitinib, abrocitinib and upadacitinib ; The only one approved in Europe for AD (and in the process of approval in the USA) is baricitinib, selective for JAK 1 and 2. The other two are selective for JAK 1. Baricitinib and upadacitinib are approved in our country for RA treatment. Research protocols are currently underway to approve baricitinib for AD in children and adults; likewise with upacitinib. These 3 drugs are considered small molecules, all of them are ingested orally in a single daily intake, presenting a rapid onset of action. So far it has been seen that JAK inhibitors provide great relief from pruritus and very quickly in patients with AD, the main limitation of quality of life.
Among the class adverse effects we can mention lymphomas, thrombosis and infections, which have been minimally reported in research protocols. Baricitinib presents eczema herpeticum and herpes zoster as an adverse effect; upadacitinib acneiform reaction, commonly seen in research protocols.
These molecules would seem to be a promising therapeutic group for patients with AD.
| Update on the treatment of cutaneous lymphomas . |
| Update on the treatment of mycosis fungoides By Prof. Dr. José Sanches (Brazil). |
Mycosis fungoides (MF) is a neoplasm that evolves into a wide range of clinical manifestations. Initially, it generally presents as barely infiltrated plaques, evolving over time to notably infiltrated and ulcerated tumor lesions. Its genesis lies in mature, atypical T lymphocytes, which initially involve the epidermis and as the condition progresses, they also affect the lymph nodes, peripheral blood and distant organs.
Regarding the treatment of MF, there are no clear guidelines. It is an incurable pathology despite the treatments we have available to date.
Treatment is based on the extent of clinical manifestations, the type of lesions present, and the presence of systemic disease. We have topical and systemic alternatives based on these previously mentioned parameters. The first group includes: topical corticosteroids, mechlorethamine, carmustine, bexarotene, imiquimod and resiquimode. In addition, both UVB and PUVA phototherapy, photodynamic therapy and radiotherapy can be mentioned as physical treatment.
As a novelty within the spectrum of topical treatment there is resiquimode . It is a Toll-R 7/8 agonist that is very useful in the initial stages of MF and has very low toxicity (local irritation). It is presented in the form of a gel, at 0.03 or 0.06%. Rook et al have proven that it has a significant improvement in 75% of the treated patients, the treated lesions all improve and even regression of untreated lesions was seen.
Among the systemic options are biological response modifying drugs such as interferon, bexarotene, acitretin, alemtuzumab, vorinostate/romidepsin and brentuximab vedotin; chemotherapy agents, such as chlorambucil, methotrexate, gemcitabine, liposomal doxorubicin, among others. These last two are the most used drugs as monotherapy options. Extracorporeal photopheresis and stem cell transplantation are also alternatives. As mentioned above, given that MF is currently an incurable neoplasm and depending on the commitment of each particular case, objective response rates with these treatments reach 30-50%.
For early stages, interferon continues to be the first therapeutic choice.
Brentuximab vedotin , a systemic alternative, is a monoclonal antibody like mogalizumab . They are being studied under research protocols, but the first would apparently have an overall remission rate of 60%.
| Update on the treatment of Sezary syndrome By Dr. Paula Enz (Argentina). |
Clinical suspicion in the context of erythroderma (involvement of more than 80% of the body surface, T4), lymph nodes and Sezary cells in peripheral blood.
> Current concept: use of multimodal therapies directed at the skin associated with rotating tandem systemic therapies (with new biological agents and immunomodulators). Cytotoxic chemotherapy only in a context of disease progression or failure in the clinical response to another established treatment.
The treatments are those mentioned previously.
Phototherapy is an excellent alternative to treat pruritus, which is usually limiting in these patients. Likewise, the electron bath, which can be combined with topical treatments, IFN or photopheresis. More than 30 Gy is effective, but is associated with significant skin toxicity, or a lower dose can be used in localized lesions. This leaves the possibility of performing shorter treatments and retracing.
Photopheresis is a good first therapeutic option. It is done 2 consecutive days every 2, 3 or 4 weeks. It can be used alone or combined with other therapies. The overall response is 40% but the studies are of few patients. It requires an “intact” immune system, so it is recommended to use it prior to immunosuppressive treatment.
Vorinostat and romidepsin are inhibitors of histone acetylation. There are no comparative studies between the two. Both have overall responses of around 35%.
Mogalizumab was approved in 2018, it is an anti-CCR4 . It has an overall response of 37% in SS. It is useful in combination with other immunomodulatory treatments.
Brentuximab is used in patients who have >10% CD30+ lymphocytes in skin biopsies from patients with SS.
Alemtuzumab has several serious adverse events (immunosuppression and infections with death), protocols are being made for SS at low doses .
Allogeneic bone marrow transplant is an alternative in patients with good general condition who have not responded to first-line treatment. It can be a healing alternative.
Monotherapy or multimodal treatments for SS? Many studies talk about combining photopheresis with immunomodulatory treatments, which has an increase in effectiveness with an overall response close to 80%. Multimodal treatment would be the appropriate course of action.
Enz commented that a patient with SS being treated with photopheresis and bexarotene had a COVID infection during 2020 at a bad time in his skin involvement. After the infection, his skin evolved very favorably.
> Future: oncolytic virotherapy, with measles viral vectors. Under study, but apparently has a good response so far.
| Treatment of primary cutaneous CD30 lymphoproliferative syndromes By Dr. Mariana Arias (Argentina). |
It includes primary cutaneous anaplastic large cell lymphoma (PLCLCL) and lymphomatoid papulosis (LP). These represent approximately 25% of primary cutaneous lymphomas, second in frequency after MF and SS.
LACGCP: adults, men (3:1), 10-year survival of 90%. Erythematous-violaceous tumors of rapid growth, which can ulcerate, generally large, in any anatomical site. Up to 40% of cases may have spontaneous regression, as well as may recur. Extracutaneous progression is common, particularly at the lymph node level, clouding the prognosis. Likewise, cases of multiple tumors in the lower limbs deserve special attention; since they usually present lymph node involvement.
PL: adults, excellent prognosis with 100% 10-year survival. High association with other lymphomas, usually with MF. It deserves close monitoring. Chronic and recurrent eruption of papules and nodules that can ulcerate, on the trunk and limbs. Autoinvolution is very common, resulting in episodes of flare-ups and remissions.
It is key to avoid overtreatment since these neoplasms have a very good prognosis. The choice is based on the extent and number of injuries. Little scientific evidence for cases of recurrence or systemic involvement.
For LACGCP:
> Single lesion or grouped lesions : surgical removal associated with localized radiotherapy or localized radiotherapy only with a margin both laterally and in depth of 2 cm. With associated lymph node involvement : localized skin and lymph node radiotherapy ± brentuximab or localized skin and lymph node radiotherapy (1st line NCCN 2021).
> Multifocal lesions : brentuximab vedotin as 1st line (NCCN 2021); As other options, an association of systemic treatments +/- treatments directed at the skin (topical or physical) is proposed.
Review of 10 publications of primary cutaneous lymphomas treated with brentuximab vedotin: 100% of patients presented a complete response in a median time of 5 weeks; reports of mild adverse effects.
For LP, observation can be made knowing that the limiting factor is the presence of a second associated lymphoma.
> Grouped lesions: phototherapy or topical corticosteroids.
> Extended lesions : methotrexate, phototherapy (NCCN 2021), systemic retinoids.
| The biological revolution in psoriasis By Prof. Dr. Jonathan Barker (United Kingdom). |
Over the past 30 years, the understanding of psoriasis has evolved incredibly. In the 80’s we considered its genesis as a disorder of epidermal differentiation, which is why immunosuppressive drugs such as methotrexate began to be used. Then, attention turned to immunology and cyclosporine began to be used here, based on a Swiss study that evaluated patients with rheumatoid arthritis treated with this drug. In the last 20 years we have aimed to stratify each particular case to offer targeted treatment, understanding that cutaneous lymphocytes synthesize a large number of cytokines that make up an intricate network that give rise to different clinical conditions. We find ourselves in the era of biological treatments. They have undoubtedly changed the course of psoriasis, but like any treatment they have their limitations. They are very expensive, they are not effective in all types of psoriasis, their effect decreases over time, of course they have their adverse effects, etc.
In the UK, biological treatments are approved by the National Health Service with strict controls. The treating teams measure the levels of the different biological drugs a month after starting the treatment since it was seen that those patients who do not reach a certain desired concentration range of the same in serum are candidates to suspend the biological and rotate the treatment for another. . They do this as a strategy to save resources knowing that these patients are more prone to primary failures due to a biological agent. Barker said they carried out adalimumab on time.
It is impossible not to mention the COVID pandemic in relation to these patients. Barker carried out records on the follow-up of patients with psoriasis treated with biologicals, so we will have these results in the future. In relation to the COVID vaccine, it is unknown how much protective effect it will have in this group of patients and how safe it is. While you have to be cautious with recommendations, he is recommending it for his patients.
Barker estimates that in the next 10 years, different topical options will emerge and the use of biomarkers in clinical practice will expand, in order to categorize patients and offer “tailored” targeted treatment.
| New diagnostic methods in leprosy By Dr. Heitor Goncalves (Brazil). |
It proposes diagnostic methods to more easily characterize different diagnostic challenges of clinical practice: indeterminate leprosy without alteration of sensitivity, differential diagnosis between reverse reaction and recurrence in paucibacillary patients, differential diagnosis between reverse reaction and recurrence in multibacillary patients, pure neural forms, Silent neural palsy and early diagnosis of both multi- and paucibacillary patients in endemic areas.
> Diagnostic tools:
Clinical THE MOST IMPORTANT (thermal sensitivity, motor compromise);
• Laboratory: Baciloscopy (especially important in multibacillary patients).
• Histopathology (differential diagnosis of recurrence or reactional pattern in MB).
• Histamine test (differential diagnosis of indeterminate forms, useful in pediatric patients to distinguish it from acromiant eczematides observed in AD).
• Electromyogram: up to 40% of patients who do not yet present clinical manifestations of neuritis present EMG involvement beforehand; dd with other pathologies that cause neuropathies; The downside is that it does not give pathognomonic changes.
• Neural ultrasound : useful to evaluate thickening, especially in cases where decompression is necessary and to perform targeted biopsies.
• Serology : anti-leprosy antibodies are measured . The higher the concentration of Ac the patient has, a greater humoral response is inferred and, therefore, a multibacillary patient is interpreted. Useful for monitoring patients living in endemic areas. A + serology does not imply that the patient is sick, but rather that he had contact with the M. leprae bacillus and, therefore, warrants close follow-up. Detection is done by ELISA method, with 98% specificity. Of little value to paucibacillary patients.
• M. leprae PCR : detects live or viable bacillus, useful for neural, indeterminate forms, to differentiate reactional phenomena of reinfection (it may be another type of bacillus) or recurrence (same bacillus) and for paucibacillary patients.
- Conventional type: amplifies both live and dead bacilli.
- Quantitative type in real time.
- Reverse transcriptase: +; means live bacillus (recurrence and not reaction).
• Nerve biopsy: sensory nerve; rarely on motor nerves (high risk of injury). Direct it with ultrasonography. PCR can be done on the nerve.
• Study of the bacillus genome: useful for population studies, to determine relapse reinfection. The possibility of developing a vaccine is being studied.