Endovascular therapy has become one of the standard treatments for acute stroke caused by large vessel occlusion. The guidelines recommend considering endovascular therapy when there is occlusion of the M1 segment (main trunk) of the middle cerebral artery or internal carotid artery and when imaging findings indicate that the size of the infarct area (also called ischemic core) is not large, as defined by an Alberta Stroke Program Early Computed Tomography Score (ASPECTS) value of at least 6 (range 0 to 10, with lower values indicating greater infarct burden), or when there is a mismatch between the ischemic central volume and the volume of the perfusion delay area.
Patients with large infarcts (e.g., those with an ASPECTS value of ≤5) have generally been excluded from clinical trials of endovascular therapy or represented in small numbers, in part due to concerns about bleeding in the infarct area after reperfusion.
A meta-analysis that included observational studies suggested that endovascular therapy may be associated with better functional outcomes and lower 90-day mortality than medical care alone in patients with an ASPECTS value of 5 or less.
Our objective was to evaluate the effect of endovascular therapy with medical care, compared with medical care alone, in patients with acute ischemic stroke caused by large vessel occlusion and a large ischemic region, defined as an ASPECTS value of 3. to 5.
We do not evaluate patients with an ASPECTS value of 2 or less because they have an extensive infarct and are unlikely to regain functional independence.
Background
Endovascular therapy for acute ischemic stroke is generally avoided when the infarct is large, but the effect of endovascular therapy with medical care compared with medical care alone for large strokes has not been well studied.
Methods
We conducted a multicenter, open-label, randomized clinical trial in Japan involving patients with cerebral large vessel occlusion and significant stroke on imaging, as indicated by an Accident Program Early Computed Tomography Score (ASPECTS) value. Alberta Cerebrovascular Diseases from 3 to 5 (on a scale of 0 to 10, with lower values indicating a larger infarct).
Patients were randomly assigned in a 1:1 ratio to receive endovascular therapy with medical care or medical care alone within 6 hours of being last known to be well or within 24 hours if there was no early change in fluid-attenuated inversion recovery images.
Alteplase (0.6 mg per kilogram of body weight) was used when appropriate in both groups. The primary outcome was a modified Rankin Scale score of 0 to 3 (on a scale of 0 to 6, with higher scores indicating greater disability) at 90 days.
Results
A total of 203 patients were randomized; 101 patients were assigned to the endovascular therapy group and 102 to the medical care group. Approximately 27% of patients in each group received alteplase.
The percentage of patients with a modified Rankin Scale score of 0 to 3 at 90 days was 31.0% in the endovascular therapy group and 12.7% in the medical care group (relative risk, 2 .43, 95% confidence interval [CI], 1.35) to 4.37; P=0.002).
The ordinal change in the range of modified Rankin scale scores generally favored endovascular therapy.
An improvement of at least 8 points in the NIHSS score at 48 hours was observed in 31.0% of patients in the endovascular therapy group and 8.8% of those in the medical care group (relative risk, 3 .51; 95% CI, 1.76). to 7.00), and some intracranial hemorrhage occurred in 58.0% and 31.4%.
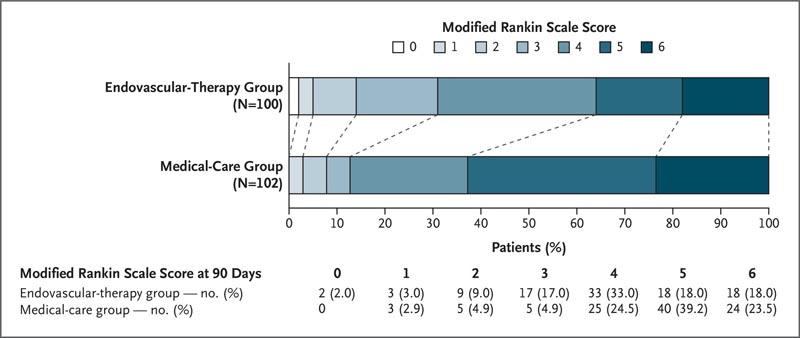
Figure : Distribution of modified Rankin scale scores at 90 days.A score of 0 on the modified Rankin scale indicates no disability, 1 no clinically significant disability, 2 mild disability, 3 moderate disability but able to walk without assistance, 4 moderately severe disability, 5 severe disability, and 6 death.
Conclusions In a trial in Japan, patients with large brain infarcts had better functional outcomes with endovascular therapy than with medical care alone, but had more intracranial hemorrhages. |
(Funded by the Mihara Cerebrovascular Disorders Research Promotion Fund and the Japan Society for Neuroendovascular Therapy; RESCUE-Japan LIMIT ClinicalTrials.gov number, NCT03702413. opens in new tab.)
















