Chronic Hypertension and Pregnancy – CHAP
Background
The benefits and safety of treating mild chronic hypertension (blood pressure <160/100 mm Hg) during pregnancy are uncertain. Data are needed on whether a strategy targeting a blood pressure of less than 140/90 mm Hg reduces the incidence of adverse pregnancy outcomes without compromising fetal growth.
Methods
In this randomized, multicenter, open-label trial, we assigned pregnant women with mild chronic hypertension and singleton fetuses with a gestational age of less than 23 weeks to receive antihypertensive medications recommended for use during pregnancy (active treatment group) or to not receive such treatment unless severe hypertension (systolic pressure, ≥160 mm Hg; or diastolic pressure, ≥105 mm Hg) developed (control group).
The primary outcome was a composite of preeclampsia with severe features, medically indicated preterm birth at less than 35 weeks’ gestation, placental abruption, or fetal or neonatal death.
The safety outcome was low birth weight for gestational age below the 10th percentile for gestational age.
Secondary outcomes included composites of severe maternal or neonatal complications, preeclampsia, and preterm birth.
Results
A total of 2,408 women were enrolled in the trial. The incidence of a primary outcome event was lower in the active treatment group than in the control group (30.2% vs. 37.0%), for an adjusted risk ratio of 0.82 (confidence interval [ 95% CI, 0.74 to 0.92; p<0.001).
The percentage of small-for-gestational-age birth weight below the 10th percentile was 11.2% in the active treatment group and 10.4% in the control group (adjusted hazard ratio, 1.04; 0 .82 to 1.31; P = 0.76).
The incidence of serious maternal complications was 2.1% and 2.8%, respectively (hazard ratio, 0.75; 95% CI, 0.45 to 1.26), and the incidence of serious neonatal complications was 2.0% and 2.6% (hazard ratio, 0.77; 95% CI, 0.45 to 1.30).
The incidence of any preeclampsia in the two groups was 24.4% and 31.1%, respectively (hazard ratio, 0.79; 95% CI, 0.69 to 0.89).
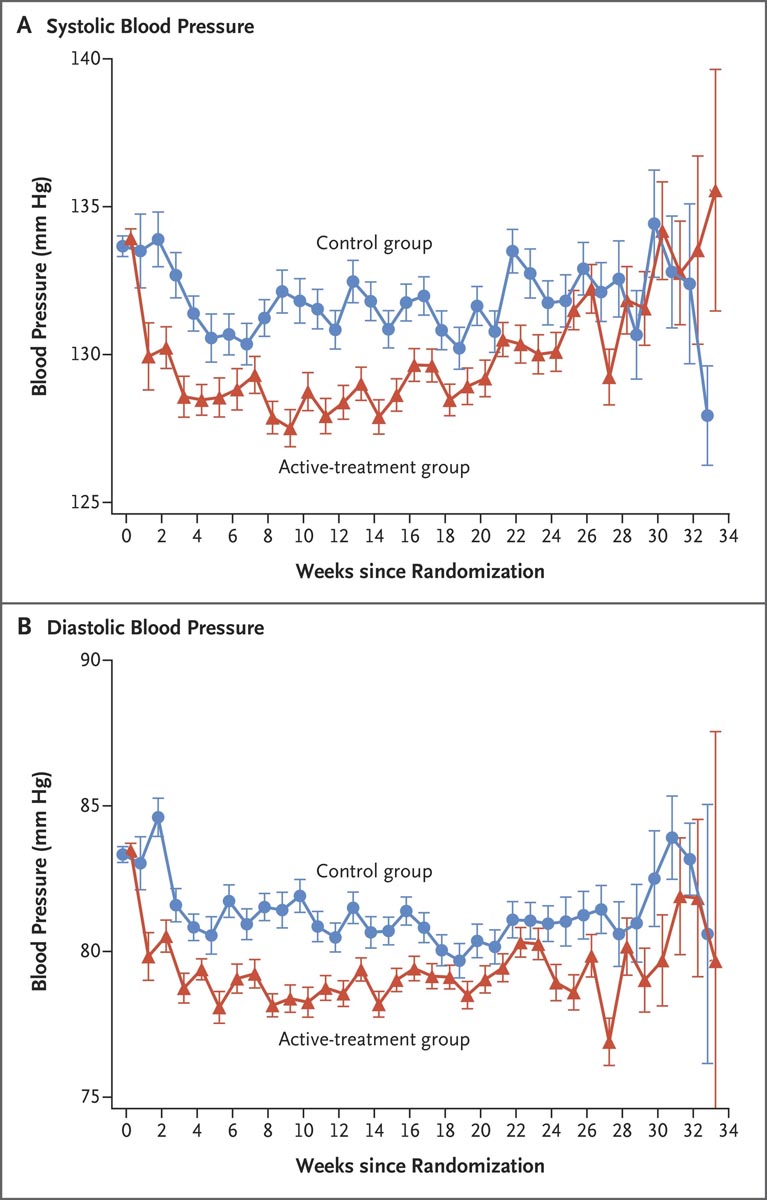
Figure: Mean arterial pressure after randomization. Between randomization and delivery, the overall mean blood pressure level was lower in the active treatment group than in the control group, both for systolic pressure (129.5 mm Hg vs. 132.6 mm Hg) and for diastolic pressure (79.1 mm Hg vs. 132.6 mm Hg). 81.5 mmHg). Bars indicate standard errors.
Conclusions In pregnant women with mild chronic hypertension, a strategy targeting a blood pressure of less than 140/90 mm Hg was associated with better pregnancy outcomes than a strategy of reserving treatment only for severe hypertension, with no increase in the risk of low weight at birth according to gestational age. |
Discussion
In pregnant women with mild chronic hypertension, active treatment with a blood pressure goal of less than 140/90 mm Hg was associated with better pregnancy outcomes than a control strategy without antihypertensive treatment, unless systolic blood pressure was outside 160 mm Hg or higher or the diastolic pressure was 105 mm Hg or higher.
Women who received active treatment had a lower risk of one or more primary outcome events of preeclampsia with severe features, medically indicated preterm birth at less than 35 weeks’ gestation, placental abruption, or fetal or neonatal death.
Estimates of components of the primary outcome and most secondary outcomes (including composites of severe maternal or neonatal complications, preeclampsia, and preterm birth) were consistent with the results of the primary analysis. It was determined that 14 to 15 patients would need to receive active treatment to prevent a primary outcome event.
There were no significant differences between groups in the safety outcome of newborns who were below the 10th percentile or the 5th percentile for gestational age weight. The difference between groups in mean arterial pressure after randomization was apparently small.
In this trial, we found that active treatment with antihypertensive medications improved pregnancy outcomes without apparent harm.
In prespecified subgroup analyzes of the primary outcome, point estimates for the hazard ratio approached 1.00 for patients with newly diagnosed chronic hypertension and for patients with a body mass index of 40 or more, but confidence intervals for 95% were large and consistent with the overall treatment effect.
The trial was not powered to assess differences in treatment effects between subgroups. Further evaluation of the treatment effect in patients with newly diagnosed hypertension or a body mass index of 40 or more may be informative. Our results suggest that the incidence of severe hypertension was lower among patients who received active treatment.
References
Tita AT, Szychowski JM, Boggess K, et al., on behalf of the Chronic Hypertension and Pregnancy (CHAP) Trial Consortium. Treatment for Mild Chronic Hypertension During Pregnancy. N Engl J Med 2022
(Funded by the National Heart, Lung, and Blood Institute; CHAP ClinicalTrials.gov number, NCT02299414. opens in new tab.)
















