European Congress of Clinical Microbiology & Infectious Diseases (ECCMID) and The Lancet.
Summary
Background
Low doses of corticosteroids have been shown to reduce mortality in COVID-19 patients requiring oxygen or ventilatory support (non-invasive mechanical ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation). The use of a higher dose of corticosteroids was evaluated in this group of patients.
Methods
This randomized, controlled, open-label platform trial (Randomized Evaluation of COVID-19 Therapy [RECOVERY]) is evaluating multiple potential treatments in patients hospitalized with COVID-19.
Eligible, consenting adult patients with clinical evidence of hypoxia (i.e., receiving oxygen or with oxygen saturation <92% on room air) were randomly assigned (1:1) to usual care with higher doses. corticosteroid discharge (dexamethasone 20 mg once daily for 5 days followed by dexamethasone 10 mg once daily for 5 days or until discharge if earlier) or usual standard of care alone (which included dexamethasone 6 mg once daily day for 10 days or until discharge if sooner).
The primary outcome was 28-day mortality among all randomized participants. On May 11, 2022, the independent data monitoring committee recommended stopping recruitment of patients receiving no oxygen or only simple oxygen due to safety concerns. We report results only for these participants. Recruitment of patients receiving ventilatory support is ongoing. The RECOVERY trial is registered with ISRCTN (50189673) and ClinicalTrials.gov (NCT04381936).
Results
Between May 25, 2021 and May 13, 2022, 1,272 patients with COVID-19 and hypoxia who received no oxygen (eight [1%]) or only simple oxygen (1,264 [99%]) were randomly assigned to receiving usual care plus low-dose corticosteroids (659 patients) versus usual care alone (613 patients, of whom 87% received low-dose corticosteroids during the follow-up period).
Of those randomized, 745 (59%) were in Asia, 512 (40%) in the UK and 15 (1%) in Africa. 248 (19%) had diabetes and 769 (60%) were men. Overall, 123 (19%) of 659 patients assigned to higher doses of corticosteroids versus 75 (12%) of 613 patients assigned to usual care died within 28 days (rate ratio 1·59 [95% CI] 1·20–2·10] ;p=0·0012).
An excess of pneumonia due to non-COVID infection was also reported (64 cases [10%] vs 37 cases [6%]; absolute difference 3.7% [95% CI: 0.7–6.6 ]) and increased hyperglycemia requiring an increase in insulin dose (142 [22%] vs 87 [14%]; absolute difference 7.4% [95% CI 3.2–11.5]) .
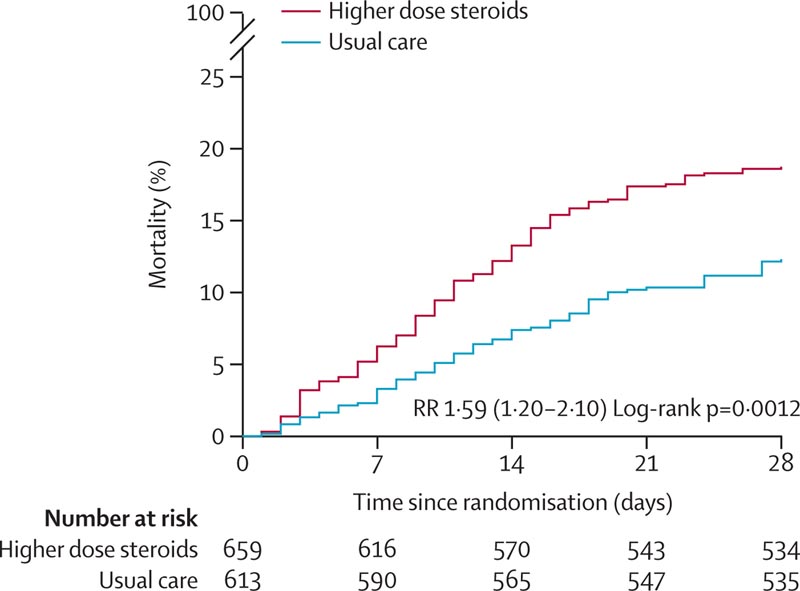
Effect of Allocation to Higher-Dose Corticosteroids or Usual Care (Lower-Dose Corticosteroids) on 28-Day Mortality in Patients Receiving No Oxygen or Only Simple Oxygen
Interpretation
In patients hospitalized for COVID-19 with clinical hypoxia who required no oxygen or only simple oxygen, higher doses of corticosteroids significantly increased the risk of death compared with usual care, which included low-dose corticosteroids.
The RECOVERY trial continues to evaluate the effects of higher doses of corticosteroids in hospitalized patients with COVID-19 requiring non-invasive ventilation, invasive mechanical ventilation, or extracorporeal membrane oxygenation.
Money
UK Research and Innovation (Medical Research Council), National Institute for Health and Care Research and the Wellcome Trust.
Comments
A new study to be presented at this year’s European Congress of Clinical Microbiology and Infectious Diseases (ECCMID 2023, Copenhagen April 15-18), and published in The Lancet , shows that, compared to standard care that included use of low doses of corticosteroids, treatment of hypoxia. COVID-19 patients who only need oxygen therapy or do not need respiratory support with higher doses of corticosteroids are associated with a 60% higher risk of death.
This study carried out by RECOVERY Collaborative Group and led by Prof. Sir Peter Horby and Prof. Sir Martin Landray (both from the University of Oxford, United Kingdom) had already identified that low doses of corticosteroids reduce the mortality of patients with COVID-19 requiring oxygen or supportive ventilation.
Since May 2021, the RECOVERY trial is evaluating the use of a higher dose of corticosteroids in this group of patients. However, in May 2022, the independent Data Monitoring Committee advised that this treatment evaluation be stopped for those patients receiving oxygen only or no respiratory support. The trial continues to study the effects of high doses of corticosteroids for those who need non-invasive or invasive mechanical ventilation.
Eligible and consenting adult patients with COVID-19 and clinical evidence of hypoxia (i.e., receiving oxygen or with an oxygen saturation <92% on normal room air) were randomly assigned (1:1) to care. with higher-dose corticosteroids (dexamethasone 20 mg once daily for 5 days followed by dexamethasone 10 mg once daily for 5 days or until discharge if sooner) or usual standard of care alone (which included dexamethasone at the lowest dose of 6 mg once daily for 10 days or until discharge if earlier). The primary outcome was 28-day mortality among all randomized participants.
Between May 25, 2021 and May 13, 2022, 1,272 patients with COVID-19 and hypoxia who received no oxygen (eight [1%]) or only simple oxygen (1,264 [99%]) were randomly assigned to receiving usual care plus low-dose corticosteroids (659 patients) versus usual care alone (613 patients, of whom 87% received low-dose corticosteroids during the follow-up period).
Of those randomized, 745 (59%) were in Asia, 512 (40%) in the UK and 15 (1%) in Africa. 248 (19%) had diabetes and 769 (60%) were men. Overall, 123 (19%) of 659 patients assigned to higher doses of corticosteroids versus 75 (12%) of 613 patients assigned to usual care died within 28 days, a 60% increase in risk of mortality for the higher dose corticosteroid group.
An excess of pneumonia due to non-COVID infection was also reported in the higher-dose corticosteroid group: 64 cases (10%) vs. 37 cases (6%); and increased hyperglycemia (episode of high blood sugar) requiring an increase in insulin dose: 142 [22%] vs. 87 [14%].
The authors conclude: “Among hospitalized patients with COVID-19 who require oxygen or ventilatory support, low doses of corticosteroids reduce the risk of death. However, among patients requiring only simple oxygen, higher doses of corticosteroids increase the risk of death compared with lower doses of corticosteroids. It is unclear whether the use of a higher dose of corticosteroids is beneficial among patients requiring noninvasive or invasive ventilation; the RECOVERY trial continues to study it.”
Added value of this study The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial is the largest randomized trial of the effect of different doses of corticosteroids in hospitalized patients with COVID-19 and included patients from three continents. We found that among patients with hypoxia on simple oxygen or without oxygen, randomization to higher doses of corticosteroids (dexamethasone 20 mg daily for 5 days followed by dexamethasone 10 mg for 5 days, or until discharge if sooner) versus care Usual treatment (which included dexamethasone 6 mg once daily in 87% of participants) resulted in an increased risk of all-cause mortality . Implications of all available evidence Among hospitalized patients with COVID-19 who require oxygen or ventilatory support, low doses of corticosteroids reduce the risk of death . However, among patients requiring only simple oxygen, higher doses of corticosteroids increase the risk of death compared with lower doses of corticosteroids. It is still unclear whether the use of a higher dose of corticosteroids is beneficial among patients requiring noninvasive or invasive ventilation; the RECOVERY trial continues to study it. |
Professor Sir Peter Horby, Professor of Emerging Infections and Global Health at the University of Oxford and Director of the Pandemic Sciences Institute, University of Oxford, UK. E) peter.horby@ndm.ox.ac.uk
Professor Sir Martin Landray, Professor of Medicine and Epidemiology, Oxford Population Health, University of Oxford, UK. E) martin.landray@ndph.ox.ac.uk