Key points What is the association of exposure to nirmatrelvir and ritonavir with the risk of COVID-19-related death or hospitalization when taking into account patient vulnerability to complications of COVID-19 infection? Findings In this cohort study of 6,866 people with COVID-19, treatment with nirmatrelvir and ritonavir was associated with a lower risk of death or hospitalization in the most clinically extremely vulnerable people, but not in the least vulnerable. People who were not extremely vulnerable to experiencing complications from COVID-19, whose average age was 79 years, were at increased risk of experiencing the outcome while receiving nirmatrelvir and ritonavir, but the finding was not statistically significant. Meaning In this study, treatment with nirmatrelvir and ritonavir was not associated with a reduced risk of death or hospitalization among people who were not extremely vulnerable to complications of COVID-19 infection, regardless of their age. |
Importance
Postmarketing analysis of people receiving nirmatrelvir and ritonavir (Paxlovid [Pfizer]) is essential because they differ substantially from people included in published clinical trials.
Aim
To examine the association of nirmatrelvir and ritonavir with the prevention of death or hospital admission in people at different risks of complications from COVID-19 infection.
Design, environment and participants
This is a cohort study of adult patients in British Columbia, Canada, between February 1, 2022 and February 3, 2023. Patients were eligible if they belonged to 1 of 4 higher-risk groups of people who received priority for vaccination against COVID-19.
Two groups included clinically extremely vulnerable (CEV) people who were severely (CEV1) or moderately immunocompromised (CEV2). CEV3 individuals were not immunocompromised but had medical conditions associated with a high risk of COVID-19 complications. A fourth expanded eligibility group (EXEL) was added to allow broader access to nirmatrelvir and ritonavir for other higher-risk people who were not in a CEV group, such as those over 70 years of age who were not vaccinated.
Exhibitions
COVID-19 patients receiving nirmatrelvir and ritonavir were matched with patients in the same vulnerability group; who were of the same sex, age, and propensity score for treatment with nirmatrelvir and ritonavir; and who also became infected within 1 month of the individual treated with nirmatrelvir and ritonavir.
Main results and measures
The primary outcome was death from any cause or emergency hospitalization with COVID-19 within 28 days.
Results
6,866 people were included in the study , of which 3,888 (56.6%) were women and whose median age (IQR) was 70 (57-80) years. Compared with unexposed controls, treatment with nirmatrelvir and ritonavir was associated with statistically significant relative reductions in the primary outcome in the CEV1 group (560 patients; risk difference [RD], −2.5%, 95% CI , −4.8% to −0.2%). ) and the CEV2 group (2628 patients; RD, −1.7%; 95% CI, −2.9% to −0.5%).
In the CEV3 group, the RD was -1.3%, but the findings were not statistically significant (2100 patients; 95% CI, -2.8% to 0.1%).
In the EXEL group, treatment was associated with an increased risk of outcome (RD, 1.0%), but the findings were not statistically significant (1578 patients; 95% CI, −0.9% to 2.9% ).
Cumulative incidence of death or emergency hospitalization related to COVID-19 . CEV indicates clinically extremely vulnerable; EXEL, expanded eligibility.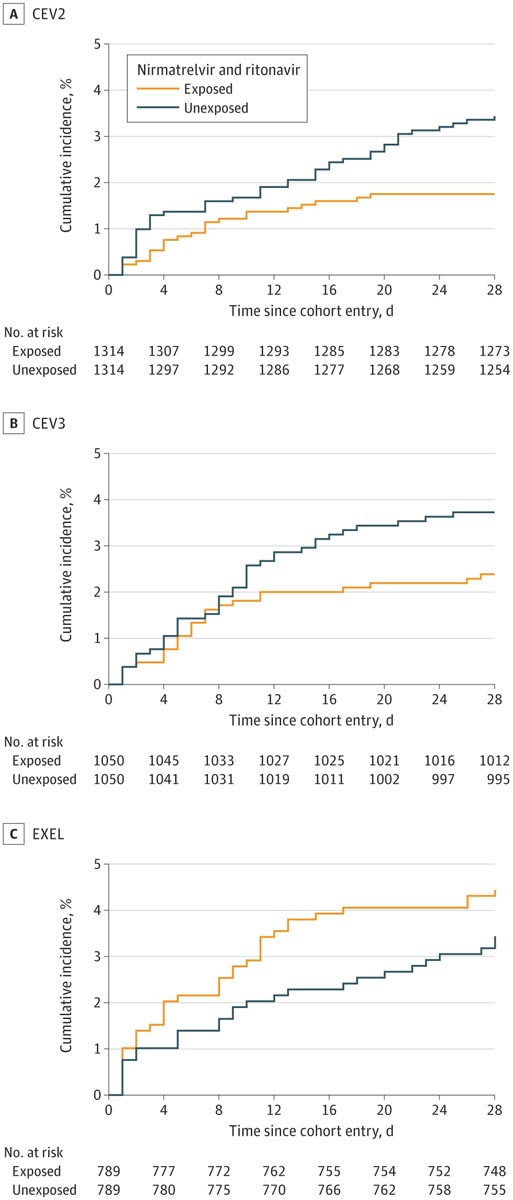
Conclusions and relevance
In this cohort study of 6,866 people in British Columbia, treatment with nirmatrelvir and ritonavir was associated with a reduced risk of hospitalization or death from COVID-19 in CEV people, with the greatest benefit seen in severely immunocompromised people.
No reduction in the primary outcome was observed in lower-risk individuals, including those aged 70 years or older without serious comorbidities.
Final message Paxlovid reduced the composite of death or emergency hospitalization in people at higher risk versus comparable controls, but not in those at lower risk/"not extremely vulnerable" (EXEL), in a Canadian study of 6,866 people. |