A study linking viral infection with reduced levels of serotonin , a neurotransmitter involved in learning, memory and mood, has proposed a new potential mechanism underlying the post-COVID-19 condition. Also known as long COVID, the condition involves symptoms such as fatigue, memory loss and cognitive decline.
Using results from human participants, mice, and organoid cultures, the researchers found that long COVID was linked to a decrease in serotonin. A viral reservoir in the intestine appeared to trigger inflammation that decreased intestinal absorption of tryptophan , the precursor molecule to serotonin.
Serotonin activity supports vagus nerve function, among other functions. In the study, loss of serotonin was associated with lower nerve activity. Dysfunction in the vagus nerve was linked to long-term symptoms characteristic of COVID, such as memory loss and hippocampal dysfunction.
“Physicians treating patients with long COVID have relied on self-reports from those patients to determine whether their symptoms are improving,” Sara Cherry, PhD, author of the study published in Cell, said in a statement. "Now, our research shows that there are biomarkers that we can use to match patients to treatments or clinical trials."
Serotonin reduction in post-acute sequelae of viral infection Highlights • Long COVID is associated with reduced levels of circulating serotonin. • Serotonin depletion is driven by viral RNA-induced type I interferons (IFNs). • IFNs reduce serotonin through decreased tryptophan absorption and hypercoagulability. • Peripheral serotonin deficiency impairs cognition through reduced vagal signaling. |
Summary
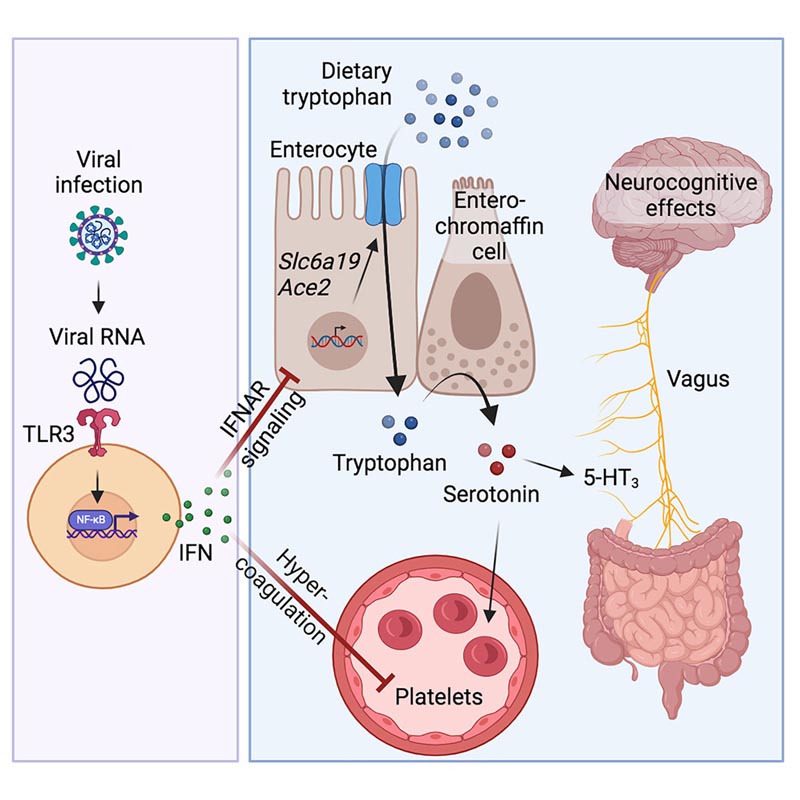
The post-acute sequelae of COVID-19 (PASC, “Long COVID”) pose a significant challenge to global health. The pathophysiology is unknown and to date no effective treatments have been found. Several hypotheses have been formulated to explain the etiology of PASC, including viral persistence, chronic inflammation, hypercoagulability, and autonomic dysfunction.
Here, we propose a mechanism that links the four hypotheses into a single pathway and provides practical insights for therapeutic interventions. We found that PASCs are associated with serotonin reduction. Viral infection and inflammation caused by type I interferon reduce serotonin through three mechanisms: decreased intestinal absorption of tryptophan, a serotonin precursor; platelet hyperactivation and thrombocytopenia, which affects serotonin storage; and increased MAO-mediated serotonin turnover.
The reduction in peripheral serotonin, in turn, impedes the activity of the vagus nerve and therefore impairs hippocampal responses and memory. These findings provide a possible explanation for the neurocognitive symptoms associated with viral persistence in long COVID, which may extend to other post-viral syndromes.
















